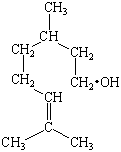
Practice Problem 6
More than 50 organic compounds have been isolated from the oil that gives rise to the characteristic odor of a rose. One of the most abundant of these compounds is known by the common name citronellol. Use the systematic nomenclature to name this alcohol, which has the following structure.

Solution
The longest chain of carbon atoms in this compound contains eight atoms.
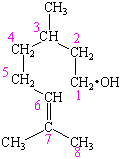
The longest chain contains the ![]() OH group,
which means this compound is named as a derivative of octane.
Because it is an alcohol, it would be tempting to name it as an octanol.
But it contains a C=C double bond, which means it must be an octenol.
We now have to indicate that the
OH group,
which means this compound is named as a derivative of octane.
Because it is an alcohol, it would be tempting to name it as an octanol.
But it contains a C=C double bond, which means it must be an octenol.
We now have to indicate that the ![]() OH group is on one end of the
chain and the C=C double bond occurs between the 6th and 7th
carbon atoms of this chain, which can be done by considering the
compound to be a derivative of 6-octen-1-ol. Finally, we
have to indicate the presence of a pair of CH3 groups
on the third and seventh carbon atom. This compound is therefore
given the systematic name 3,7-dimethyl-6-octen-1-ol.
OH group is on one end of the
chain and the C=C double bond occurs between the 6th and 7th
carbon atoms of this chain, which can be done by considering the
compound to be a derivative of 6-octen-1-ol. Finally, we
have to indicate the presence of a pair of CH3 groups
on the third and seventh carbon atom. This compound is therefore
given the systematic name 3,7-dimethyl-6-octen-1-ol.