CHAPTER 11:
AClDS AND BASES
The following information is often presented on the first page of exams covering this chapter under the heading: “potentially useful information.”
[X] = concentration of X at equilibrium, in moles per liter
(X) = concentration of X at any moment in time, in moles per liter
CX = initial concentration of X, in moles per liter
Acids, Bases, and Salts
11-1. Which of the following groups contains salts that all form basic solutions in water?
(a) NaNO3, NH4CN, NaOAc, NH4Cl
(b) Na2CO3, KCl, NaOAc, NH4Cl
(c) Na2CO3, NaF, NaOAc, NaCN
(d) NaHCO3, NaF, NH4Cl, Na2SO3
(e) none of the above
Answer: (c)
11-2. Which of the following compounds would give an 0.10 M solution with a pH less than 7?
(a) Na2S (b) K2CO3 (c) KCN (d) NH4Cl (e) Na3PO4
Answer: (d)
11-3. Which of the following is a strong acid?
(a) HNO3 (b) H2S (c) HNO2 (d) HCO3- (e) HOCl
Answer: (a)
11-4. Which of the following is a strong electrolyte?
(a) Ca(CN)2 (b) NH3 (c) HCN (d) H2S (e) acetic acid
Answer: (a)
11-5. Which of the following compounds is not an Arrhenius acid?
(a) HCl (b) H2SO4 (c) H2O (d) FeCl3 (e) H2S
Answer: (d)
11-6. Which of the following compounds is not an Arrhenius base?
(a) NaOH (b) MgO (c) Ca(OH)2 (d) H2O (e) NH3
Answer: (e)
11-7. The following compounds dissolve in water to form solutions that turn litmus from red to blue. Which of these compounds satisfy the operational definition of a base?
(a) NaHCO3 (b) CaO (c) CaH2 (d) NH3 (e) all of the above
Answer: (e)
11-8. What is the H3O+ ion concentration in a solution that has a pH of 5.75?
(a) 5.8 x 10-5 (b) 3.2 x 10-3 (c) 0.75 x 10-5 (d) 1.8 x 10-6
Answer: (d)
11-9. Element X reacts with excess oxygen to form a basic oxide with the formula XO. In which Group of the periodic table should X be placed?
(a) Group IIA (b) Group IIIA (c) Group IVA (d) Group VIA (e) Group VIIA
Answer: (a)
11-10. If the element described in the previous question were to react with hydrogen it should form:
(a) an acidic hydride, XH (b) an acidic hydride, XH2
(c) a basic hydride, XH (d) a basic hydride, XH2
(e) none of the above
Answer: (d)
11-11. Which Group IVa element is most likely to form an oxide with the formula XO2 that dissolves in water to form an acidic solution?
(a) C (b) Si (c) Ge (d) Sn (e) Pb
Answer: (a)
11-12. Which of the following elements would be the most likely to form an acidic oxide with the formula XO2 and an acidic hydride with the formula XH2?
(a) Na (b) Mg (c) Al (d) S (e) Cl
Answer: (d)
11-13. Which compound should dissolve in water to form an 0.1 M solution with a pH of 8?
(a) HCl (b) NaOAc (CH3CO2Na) (c) HOAc (CH3CO2H)
(d) NaCl (e) KOH
Answer: (b)
11-14. Which of the following compounds exhibits the following behavior. It dissolves in water to give a colorless, odorless solution with a pH of 11. This solution reacts with HCl to form an odorless gas. When AgNO3 is added to this solution, a yellow precipitate is formed.
(a) H2SO4 (b) Cu(OH)2 (c) Na2CO3 (d) Na2S (e) NaCl
Answer: (c)
11-15. Which of the following acid-base reactions should go more or less to completion?
(a) HClO4(aq) + OCl-(aq) ➝ ClO4-(aq) + HOCl(aq)
(b) HF(aq) + I-(aq) ➝ HI(aq) + F-(aq)
(c) H2SO3(aq) + SO42-(aq) ➝ H2SO4(aq) + SO32-(aq)
(d) H2S(aq) + 2 Cl-(aq) ➝ S2-(aq) + 2 HCl(aq)
(e) All of these reactions would go to completion, giving high yields of products.
Answer: (a)
11-16. Which of the following won’t produce an acidic solution when dissolved in water? (a) FeCl3 (b) NH4Cl (c) acetic acid (CH3CO2H)
(d) sodium acetate (CH3CO2Na) (e) HClO4
Answer: (d)
11-17. Which of the following statements is correct?
(a) nonmetals usually form basic oxides
(b) metals usually form basic hydroxides
(c) nonmetals have the lowest electronegativities
(d) nonmetals usually form ionic compounds with other nonmetals
(e) all of the above are correct.
Answer: (b)
11-18. Which of the following gives a basic solution when dissolved in water?
(a) CaO (b) CO2 (c) Cl2O (d) SO2 (e) SeO3
Answer: (a)
11-19. Which of the following compounds isn’t an acid in water?
(I) SO2 (II) HNO3 (III) SrO (IV) HI (V) K2S
(a) I and II (b) II and III (c) III and V
(d) I, II and IV (e) all of the above are acids in water
Answer: (c)
11-20. Which of the following compounds isn’t a base in water?
(a) Ca(OH)2 (b) CuO (c) NO2 (d) Li2O (e) CsOH
Answer: (c)
11-21. Which of the following is the most likely to be a white solid with a high melting point that dissolves in water to form a basic solution?
(a) O2 (b) CO2 (c) Na2O (d) P4O10 (e) Cl2O7
Answer: (c)
11-22. Which of the following oxides would yield the most basic solution when dissolved in water?
(a) ClO2 (b) SiO2 (c) SO2 (d) Al2O3
Answer: (d)
11-23. Which of the following isn’t an acid-base reaction.
(a) 2 NaCl(s) + MnO2(s) + 4 H+(aq) ➝ 2 Na+(aq) + Mn2+(aq) + H2O(l) + Cl2(g)
(b) 2 NaCl(s) + H2SO4(aq) ➝ Na2SO4(aq) + 2 HCl(g)
(c) Na2CO3(aq) + H2SO4(aq) ➝ Na2SO4(aq) + 2 H2O(l) + CO2(g)
(d) H2C2O4(aq) + 2 NaOH(aq) ➝ Na2C2O4(aq) + 2 H2O(l)
(e) all of the above are acid-base reactions
Answer: (a)
Brønsted Acids and Bases
11-24. Consider the following reaction:
H2PO4-(aq) + HCO3-(aq) ➝ H2CO3(aq) + HPO42-(aq)
Brønsted would identify the acidic species as:
(a) H2PO4- and H2CO3 (b) H2PO4- and HPO42-
(c) HCO3- and H2CO3 (d) HCO3- and HPO42-
(e) H2CO3 and HPO42-
Answer: (a)
11-25. Label the Brønsted acids and bases in the following reaction.
HSO4-(aq) + H2O(l) ➝ H3O+(aq) + SO42-(aq)
Answer: acids = HSO4- and H3O+; bases = H2O and SO42-
11-26. Which compound cannot act as a Brønsted base?
(a) NH3 (b) H2O (c) CH4 (d) Cl- (e) H3O+
Answer: (c)
11-27. Which of the following compounds cannot be an base?
(a) H2O (b) NH3 (c) CO32- (d) OH- (e) NH4+
Answer: (e)
11-28. Which of the following compounds cannot be a Brønsted base?
(a) OH- (b) H2O (c) NH3 (d) NH4+
(e) all of the above are Brønsted bases
(e) all of these compounds can be Brønsted bases.
Answer: (d)
11-29. Which of the following compounds won’t be a base in solution?
(a) NH3 (b) CaO (c) Ca(OH)2 (d) NaOH (e) NH4NO3
Answer: (e)
11-30. Which of the following isn’t a Brønsted base?
(a) PF5 (b) H2O (c) NH3 (d) S2- (e) CN-
Answer: (a)
11-31. Which of the following compounds cannot be a Brønsted base?
(a) H2O (b) MnO4- (c) BH4- (d) CN- (e) S2-
Answer: (c)
11-32. Which of the following compounds cannot be a Brønsted base?
(a) O2 (b) CO2 (c) PH3 (d) SF4 (e) CH3+
Answer: (e)
11-33. Which of the following compounds can act as either a Brønsted acid or a Brønsted base?
(I) NaHCO3 (II) Na2CO3 (III) H2CO3 (IV) CO2 (V) H2O
(a) I (b) I and II (c) II and III (d) I and V (e) V
Answer: (d)
11-34. Which compound is the most likely to be amphoteric?
(a) Na2O (b) CaO (c) Al2O3 (d) P4O10 (e) Cl2O7
Answer: (c)
11-35. Predict the products of the following acid-base reaction.
NaOCH3(aq) + NaHCO3(aq) ➝
Answer: CH3OH and CO32-
11-36. Predict the products of the following acid-base reaction.
HOCl(aq) + CH3NH2(aq) ➝
Answer: CH3NH3+ and OCl-
11-37. Predict the products of the following acid-base reaction.
NH4Cl(aq) + NaSH(aq) ➝
Answer: H2S and NH3
Conjugate Acid/Base Pairs
11-38. Which of the following is the conjugate acid of the HPO42- ion?
(a) H3PO4 (b) H2PO4- (c) HPO42- (d) PO43- (e) H3O+
Answer: (b)
11-39. What is the conjugate base of the HPO42- ion?
(a) PO43- (b) HPO42- (c) H2PO4- (d) H3PO4 (e) none of the above
Answer: (a)
11-40. What is the conjugate base of HSO4-?
(a) H2O (b) H2SO4 (c) HSO4- (d) SO3 (e) none of the above
Answer: (e)
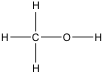
11-41. Methanol is described by the following skeleton structure.

Which compound is formed when CH3OH acts as a Brønsted base?
(a) CH3O- (b) CH3OH (c) CH3OH2+ (d) CH3OH (e) H3O+
Answer: (c)
11-42. Which of the following is the conjugate base of a strong acid?
(a) OH- (b) HSO4- (c) NH2- (d) S2- (e) H3O+
Answer: (b)
11-43. “Cocaine” as it is shown on television is actually a salt known as cocaine hydrochloride, [C17H22NO4+][Cl-], which forms white crystals that stupid people snort through straws to make them even more stupid. Recently, a lot of attention has been paid to a frightening substance known as “crack”, which is formed by treating cocaine with sodium bicarbonate, NaHCO3. “Crack” is a neutral compound with the formula C17H21NO4. Which of the following statements is true?
(a) “Crack” is the conjugate base of cocaine hydrochloride.
(b) “Cocaine” contains the conjugate acid of “crack”.
(c) When “cocaine” is treated with NaHCO3, the bicarbonate ion acts as a base, removing an H+ ion.
(d) “Crack” can be “smoked” because it is a covalent compound that becomes reasonably volatile when heated, but “cocaine” must be snorted because it is a salt that does not easily boil even when heated.
(e) All of the above are true.
Answer: (e)
11-44. Which of the following represents a conjugate acid/base pair?
(a) H+, OH- (b) H3O+, OH- (c) NH3, NH4+ (d) NaOH, HCl
(e) all of the above are conjugate acid/base pairs
Answer: (c)
11-45. Which of the following isn’t a conjugate acid/base pair?
(a) NH4+(aq) and NH3(aq) (b) H2O(l) and OH-(aq)
(c) H3O+(aq) and OH-(aq) (d) NH3(aq) and NH2-(aq)
(e) all of these are conjugate acid/base pairs.
Answer: (c)
11-46. Which of the following isn’t a Brønsted conjugate acid/base pair?
(a) NH3/NH2- (b) CH3CO2H/CH3CO2- (c) H2O/O2- (d) H3O+/H2O
Answer: (c)
Relative Strength of Acids and Bases
11-47. Which of the following is the strongest Brønsted acid?
(a) NH2- (b) HS- (c) H2O (d) CH4 (e) NH4+
Answer: (e)
11-48. Which of the following is the strongest Brønsted acid?
(a) NH4 (b) HCl (c) HNO3 (d) HSO4- (e) HClO4
Answer: (e)
11-49. Which of the following is the strongest Brønsted acid?
(a) CsH (b) H2O (c) HF (d) H2Te (e) HI
Answer: (e)
11-50. Which of the following would be the strongest Brønsted acid?
(a) H3O+ (b) HF (c) NH3 (d) NaHSO4 (e) NaOH
Answer: (a)
11-51. Which of the following would be the weakest Brønsted acid?
(a) H2Se (b) H2S (c) H2O (d) SH- (e) OH-
Answer: (e)
11-52. Which of the following would be the weakest Brønsted acid?
(a) HClO4 (b) H3PO4 (c) H2CO3 (d) H2SiO4 (e) Al(OH)3
Answer: (e)
11-53. Which of the following is the weakest acid?
(a) HClO4 (b) HCl (c) HF (d) HI (e) HBr
Answer: (c)
11-54. Which of the following is the weakest Brønsted acid?
(a) H2SO3, Ka = 0.3 (b) H2CrO4, Ka = 9.6
(c) H3BO3, Ka = 7.3 x 10-10 (d) C6H5OH, Ka = 1.0 x 10-10
Answer: (d)
11-55. Which of the following is the strongest base?
(a) I- (b) NO3- (c) HS- (d) O2- (e) OH-
Answer: (d)
11-56. Which of the following is the strongest base?
(a) NH2- (b) PH2- (c) CH3- (d) SiH3- (e) OH-
Answer: (c)
11-57. Which of the following is the strongest base?
(a) H2O (b) OH- (c) NH2- (d) NH3 (e) CH4
Answer: (c)
11-58. Of the following, which is the strongest base?
(a) F- (b) OH- (c) H2O (d) Cl- (e) O2-
Answer: (e)
11-59. Which of the following would be the strongest Brønsted base?
(a) HSO3- (b) HSO4- (c) H2SO3 (d) SO32- (e) SO42-
Answer: (d)
11-60. Which of the following would be the strongest Brønsted base?
(a) H2O (b) SH- (c) OH- (d) S2- (e) O2-
Answer: (e)
11-61. Which of the following would be the strongest Brønsted base?
(a) InH2- (b) SnH3- (c) SbH2- (d) The- (e) I-
Answer: (b)
11-62. Which oxide would dissolve in water to produce the most basic solution?
(a) CaO (b) Ga2O3 (c) GeO2 (d) P4O10 (e) SO3
Answer: (a)
11-63. Which of the following industrial compounds is the strongest base?
(a) NH4NO3 (b) (NH4)2SO4 (c) Na2CO3 (d) CO2
Answer: (c)
11-64. Which of the following is a weak base?
(a) (CH3)3N (b) HF (c) KOH (d) Na (e) H2
Answer: (a)
11-65. Which of the following would form the most basic 0.100 M solution?
(a) NaHSO4 (b) NaHCO3 (c) NaH2PO4 (d) Na2CO3 (e) NaCl
Answer: (d)
11-66. Which of the following would have the strongest conjugate base?
(a) H2O (b) H2S (c) NH3 (d) PH3 (e) CH4
Answer: (e)
11-67. Arrange the following bases in order of increasing strength: NH3, PH3, H2O and H2S.
Answer: H2S < H2O < PH3 < NH3
11-68. Which of the following is correct?
(a) K2SO3 is a stronger base than KHSO3
(b) K2CO3 is a weaker base than KHCO3
(c) NaHSO3 is a stronger acid than NaHSO4.
(d) Na2HPO4 is a weaker base than NaH2PO4.
(e) All of these are correct
Answer: (a)
11-69. Which of the following statements is true?
(a) H2O is a stronger acid than HF(aq)
(b) H2O is a stronger acid than H2S(aq)
(c) H2O is a stronger acid than the H3O+(aq) ion
(d) H2O is a stronger acid than NH3(aq)
(e) None of the above are true
Answer: (d)
11-70. Which of the following statements is true?
(a) H2O is a weaker acid than NH3
(b) PH3 is a weaker acid than NH3
(c) OH- is a weaker base than H2O
(d) NH2- is the conjugate acid of NH3
(e) ClO- is a stronger base than ClO4-
Answer: (e)
11-71. What can be correctly concluded from the fact that the following the acid-base reaction proceeds to the right, as written?
NH2-(aq) + HSO4-(aq) ➝ NH3(aq) + SO42-(aq)
(a) NH3 is a stronger base than NH2-
(b) NH3 is a stronger acid than HSO4-
(c) NH3 is a weaker acid than NH2-
(d) NH3 is a weaker base than HSO4-
(e) NH3 is a weaker acid than HSO4-
Answer: (e)
11-72. NaHCO3 can be used to neutralize strong bases, such as NaOH. What conclusion can be drawn from the fact that the following acid-base reaction proceeds to the right as written?
HCO3-(aq) + OH-(aq) ➝ CO32-(aq) + H2O(l)
(a) HCO3- is a stronger acid than H2O
(b) HCO3- is a stronger base than CO32-
(c) HCO3- is a stronger base than OH-
(d) CO32- is a stronger base than OH-
(e) H2O is a stronger acid than HCO3-
Answer: (a)
11-73. What can we conclude from the fact that the following reaction proceeds as written?
NaNH2(s) + H2O(l) ➝ NH3(aq) + OH-(aq)
(a) OH- is a stronger base than NH2-
(b) NH3 is a stronger acid than H2O
(c) NH2- is a stronger acid than H2O
(d) OH- is a weaker base than NH2-
(e) More than one of the above is true
Answer: (d)
Lewis Acids and Bases
11-74. Which compound cannot accept a pair of electrons and function as a Lewis acid?
(a) SO3 (b) BF3 (c) Ag+ (d) CH4 (e) H+
Answer: (d)
General Acid-Base Questions
Use the following acid-dissociation equilibrium constants for questions 75-77.
HA: Ka = 1.00 x 10-4 HB: Ka = 1.00 x 10-7
HC: Ka = 1.00 x 10-10 HD: Ka = 1.00 x 10-11
11-75. Solutions of each acid are prepared in which the initial concentration of the acid is 0.1000 M. Which of the four solutions will be the most acidic?
(a) HA (b) HB (c) HC (d) HD
(e) not enough information is provided to answer this question.
Answer: (a)
11-76. 0.5 M solutions of NaA, NaB, NaC, and NaD are prepared. Which solution is the most basic?
(a) NaA (b) NaB (c) NaC (d) NaD
(e) all of the solutions have the same pH.
Answer: (d)
11-77. A solution is prepared with initial concentrations of HA and NaA of 0.50 M and 1.00 M respectively. What is the pH of the solution?
(a) 3.70 (b) 4.00 (c) 4.30 (d) 9.70 (e) 10.30
Answer: (c)
11-78. Which of the following solutions would have a pH greater than 7.0?
(a) NH4Cl (b) NaCl (c) HOAc (d) NaOAc
Answer: (d)
11-79. Which of the following solutions has a pH above 7.0?
(a) 0.10 M NH4Cl (b) 0.10 M NH4NO3 (c) 0.10 M HCN
(d) 0.10 M NaCN (e) none of these
Answer: (d)
11-80. Which of the following negative ions would be the strongest base?
(a) Cl2CHCO2- (Cl2CHCOC2H: Ka = 7.8 x 10-3)
(b) ClCH2CO2- (ClCH2CO2H: Ka = 1.4 x 10-3)
(c) HCO2- (HCO2H: Ka = 1.8 x 10-4)
(d) CH3CO2- (CH3CO2H: Ka = 1.8 x 10-5)
Answer: (d)
11-81. Which of the following acids would have the strongest conjugate base?
(a) HOCl (Ka = 2.9 x 10-8)(b) HOBr (Ka = 2.4 x 10-9)
(c) HOI (Ka = 2.3 x 10-11)(d) H2O2 (Ka = 2.2 x 10-12)
(e) H2O (Ka = 1.8 x 10-16)
Answer: (e)
11-82. Which of the following solutions would be the most acidic?
(a) 0.10 M CH3CO2H (Ka = 1.8 x 10-5) (b) 0.10 M HCO2H (Ka = 1.8 x 10-4)
(c) 0.10 M ClCH2CO2H (Ka = 1.4 x 10-3) (d) 0.10 M Cl2CHCO2H (Ka = 5.1 x 10-2)
(e) All of these solutions would have the same pH.
Answer: (d)
11-83. Which of the following is the strongest Brønsted acid?
(a) NH4+ (b) HCl (c) HNO3 (d) HSO4- (e) HClO4
Answer: (e)
11-84. Which of the following is the strongest base?
(a) I- (b) NO3- (c) HS- (d) O2- (e) OH-
Answer: (d)
11-85. Which of the following is the strongest acid?
(a) NH2- (b) HS- (c) H2O (d) CH4 (e) NH4+
Answer: (e)
11-86. Explain the following observation: Water becomes acidic when CO2 is bubbled into it.
Answer: Because of the formation of carbonic acid, H2CO3
11-87. If the strength of the following acids increases from left to right
CH3NH2 < CH3NH3+ < C6H5NH3 + < C6H5CO2H < HCl
the order of increasing base strength, reading from left to right, must be:
(a) CH3NH- < CH3NH2 < C6H5NH2 < C6H5CO2- < Cl-
(b) Cl- < C6H5CO2- < C6H5NH2 < CH3NH2 < CH3NH-
(c) C6H5NH2 < CH3NH2 < Cl- < C6H5CO2- < CH3NH-
(d) CH3NH- < C6H5CO2- < Cl- < CH3NH2 < C6H5NH2
(e) none of the above
Answer: (b)
11-88. What is the pH of a solution that is 1.7 x 10-4 M in H+?
(a) 3.77 (b) 4.77 (c) 4.23 (d) 10.23 (e) none of these
Answer: (a)
11-89. What is the hydroxide ion concentration in a pH = 5.14 solution?
(a) 1.0 x 10-14 (b) 1.4 x 10-9 (c) 1.0 x 10-7 (d) 7.2 x 10-6 (e) 1.4 x 10-2
Answer: (b)
[Note: 30% of the population chose answer d, for which there was a strong negative correlation, r = -0.43, while 60% chose answer b, with a strong positive correlation, r = 0.47.]
11-90. Which of the following solutions has the largest pH?
(a) 0.3 M Na2CO3 (b) 1 M HOAc (c) 0.3 M NH4Cl
(d) water (e) 10-3 M HCl
Answer: (a)
11-91. The addition of sodium formate (HCO2Na) to a solution containing formic acid (HCO2H: Ka = 1.8 x 10-4) will cause:
(a) the pH to increase (b) the pH to decrease (c) no change in pH
(d) a change in the pH, the direction of which cannot be predicted.
Answer: (a)
Strong Acids
11-92. Which equation best describes an 0.10 M solution of a strong acid, such as hydroiodic acid (HI: K = 3 x 109)?
(a) [H3O+]T ≈ CHI (b) [H3O+]T ≈ [OH-]W (c) [H3O+]T ≈ [HI]
(d) [H3O+]T ≈ [H2O] (e) [H3O+]T ≈ KaCa
Answer: (a)
Weak Acids: General
11-93. A solution is known to have a hydronium ion concentration of 1.0 x 10-6 M. What percentage of the total H3O+ ion concentration comes from the dissociation of water?
(a) 1% (b) 3% (c) 5% (d) 10% (e) 100%
[Note: less than 30% of the sample population got the correct answer: (a) ]
Answer: (a)
11-94. Assume a solution is prepared by adding 1.0 x 10-3 moles of a weak monoprotic acid (Ka = 2.0 x 10-4) to enough water to give a liter of solution. If you want to compute the H3O+ ion concentration with an error of 5% or less, you can legitimately ignore:
(a) both the contribution of the dissociation of water and the additive ΔC term.
(b) neither the dissociation of water nor the additive ΔC term.
(c) the dissociation of water but not the additive ΔC term.
(d) the additive ΔC term but not the dissociation of water.
(e) you can't compute the H3O+ concentration with an error of less than 5%.
Answer: (c)
11-95. When 1.0 x 10-5 mole of HOCl (Ka = 3.5 x 10-8) is dissolved in pure water and diluted to 1.00 L, which assumption can't be applied in the calculation of the pH of this solution? (a) that the initial concentration of HOCl is much larger than the total H3O+ ion concentration
(b) that the H3O+ ion concentration is much larger than Kw/[H3O+]
(c) that the total H3O+ ion concentration is the sum of the concentrations from the dissociation of HOCl and water.
(d) all of these assumptions are valid.
(e) none of these assumptions are valid.
Answer: (b)
11-96. When can we ignore the contribution to the total H3O+ ion concentration from the dissociation of water?
(a) when KaCa < 1.0 x 10-13 (b) when KaCa = 1.0 x 10-13
(c) when KaCa > 1.0 x 10-13 (d) whenever we feel like it.
(e) when the cows come home.
Answer: (c)
11-97. An 0.0024 M solution of boric acid (H3BO3: Ka = 5.8 x 10-10) is an example of which kind of problem?
(a) a strong acid.
(b) a weak acid where we can ignore the dissociation of water and assume that ΔC is small compared with Ca.
(c) a weak acid where we can ignore the dissociation of water but we can't assume that ΔC is small compared with Ca.
(d) a weak acid where we can't ignore the dissociation of water but we can assume that ΔC is small compared with Ca.
(e) a weak acid where we can't ignore the dissociation of water and we can't assume that ΔC is small compared with Ca.
Answer: (b)
11-98. For a particular weak acid, HA, in pure water, as the initial concentration of the weak acid increases, the acid-dissociation equilibrium constant of that acid should:
(a) increase (b) decrease (c) remain the same
(d) increase until the ionization of water can be neglected.
(e) decrease until the ionization of water can be neglected.
Answer: (c)
11-99. What would happen if more formic acid (HCO2H) was added to the following solution at equilibrium?
HCO2H(aq) + H2O(l) ⇌ H3O+(aq) + HCO2-(aq)
(a) [H2O] should increase.
(b) [H3O+] and [HCO2-] should both increase.
(c) [H3O+] and [HCO2- should both decrease.
(d) [H3O+] should increase but [HCO2-] should decrease.
(e) [H3O+] should decrease but [HCO2-] should increase.
Answer: (b)
11-100. Which of the following equations is valid for a 0.10 M solution of formic acid, HCO2H?
HCO2H(aq) + H2O(l) ⇌ HCO2-(aq) + H3O+(aq) Ka = 1.8 x 10-4
(a) [H3O+]T ≈ [OH-]W (b) [H3O+]W ≈ [HCO2-] (c) [H3O+]T ≈ [HCO2-]
(d) [H3O+]T = [H2O] (e) [OH-] ≈ [H2O]
Answer: (c)
11-101. The value of the equilibrium constant, Ka for the dissociation of formic acid in water,
HCO2H(aq) + H2O(l) ⇌ HCO2-(aq) + H3O+(aq)
would depend on:
(a) the temperature (b) the pressure (c) the pH
(d) the concentration of HOAc
(e) the concentration of the OAc- ion.
Answer: (a)
11-102. Which of the following isn’t for the H3O+ ion concentration in an 0.0100 M solution of acetic acid (Ka = 1.8 x 10-5)?
(a) it is less than the H3O+ concentration in an 0.0100 M solution of HCl.
(b) it is equal to the OAc- ion concentration.
(c) it is equal to the OH- ion concentration.
(d) the pH of the solution is less than 7.0.
(e) none of the above are true.
Answer: (c)
11-103. Which of the following isn’t for solutions of acetic acid (Ka = 1.8 x 10-5)?
(a) the percent ionization increases as solutions are made more dilute.
(b) the [H3O+] increases as solutions are made more dilute.
(c) in a 0.100 M solution of the acid, it is approximately one percent dissociated.
(d) its percent ionization is smaller in a 0.100 M solution containing sodium acetate than in pure water.
(e) the ionization constant is independent of concentration.
Answer: (b)
Weak Acids: Calculations
11-104. What is the percent ionization in an 0.01 M HCN solution? (HCN: Ka = 4 x 10-10) (a) 0.002 % (b) 0.02% (c) 0.2% (d) 2% (e) 20%
Answer: (b)
11-105. Calculate the pH of an 0.10 M lactic acid solution. (Ka = 8.4 x 10-4)
(a) ≈2 (b) ≈3 (c) ≈4 (d) ≈7 (e) ≈12
Answer: (a)
11-106. Calculate the pH of a 0.100 M solution of formic acid (HCO2H) in water. (HCO2H: Ka = 1.8 x 10-4)
(a) 2.4 (b) 3.7 (c) 4.7 (d) 7 (e) 11.6
Answer: (a)
11-107. The very first disinfectant used by Joseph Lister was called “carbolic acid”. This substance is now known as phenol (PhOH). What is the H3O+ ion concentration in an 0.10 M solution of phenol? (PhOH: Ka = 1.0 x 10-10)
(a) 5.5 (b) 8.5 (c) 10 (d) 11 (e) none of the above
Answer: (a)
11-108. Hydrogen peroxide has been used as a bleach to change hair color, as a disinfectant to treat wounds, and as a rocket fuel. It is also a weak acid. Calculate the pH of an 0.018 M H2O2 solution. (H2O2: Ka = 2.2 x 10-12)
(a) 0.6 (b) 6.7 (c) 7.3 (d) 11.7 (e) 13.4
Answer: (b)
11-109. A 0.10 M solution of a weak acid, HA, is found to be 1.50% ionized. Calculate Ka for this acid.
(a) 1.5 (b) 2.3 x 10-1 (c) 2.3 x 10-5 (d) 2.3 x 10-6
(e) none of the above
Answer: (c)
11-110. A solution is prepared by dissolving 1.0 x 10-4 moles of HOBr in water and diluting to 1.00 L. What is the pH of this solution? (HOBr: Ka = 2 x 10-9)
(a) 1.3 (b) 5.3 (c) 6.3 (d) 8.7 (e) 12.7
Answer: (c)
11-111. A solution is prepared by dissolving 1.0 x 10-4 moles of HOBr in water and diluting to 1.00 L. What is the pH of this solution? (HOBr: Ka = 2 x 10-9)
(a) 5.3 (b) 6.3 (c) 7 (d) 7.7 (e) 8.7
Answer: (b)
Bases: General
11-112. What is the value of Kb for the formate ion if Ka for formic acid is 1.8 x 10-4?
(a) Kb = Kw x Ka (b) Kb = Ka/Kw (c) Kb = Kw/Ka
(d) Kb = Kw + Ka (e) Kb = Kw - Ka
Answer: (c)-
11-113. The base-ionization constants for three bases, A(OH), B(OH), and C(OH) are 1.8 x 10-5, 4.4 x 10-4, and 7.4 x 10-4, respectively. Solutions of the bases having equal molarity have pH values that decrease in the following order.
(a) A > B > C (b) B > C > A (c) C > B > A
(d) A > C > B (e) B > A > C
Answer: (c)
11-114. Which is the correct relation between Ka for an acid and Kb for its conjugate base?
(a) Kb = Kw x Ka (b) Kb = Kw/Ka (c) Kb = - log Ka
(d) Kb = 1/Ka (e) Kb = Ka/Kw
Answer: (b)
Bases: Calculations
11-115. Ammonia and the ammonium ion form a conjugate acid-base pair.
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH-(aq)
Calculate the pH of 0.10 M NH3 if Ka for the NH4+ ion is 5.6 x 10-10
(a) 4.7 (b) 5.1 (c) 5.7 (d) 8.9 (e) 11.1
Answer: (e)
11-116. There are many ways of “fluoridating” water. One approach involves adding a salt of the fluoride ion, such as NaF. Calculate the pH of an 0.15 M NaF solution. (HF: Ka = 7.2 x 10-4)
(a) 2 (b) 5.8 (c) 8.2 (d) 10.9 (e) 12
Answer: (c)
11-117. Calculate the pH of a solution prepared by dissolving 1.00 x 10-2 moles of sodium hypochlorite (NaOCl) in enough water to produce a liter of solution. Hypochlorous acid is a weak monoprotic acid with Ka = 3.2 x 10-8.
(a) 4.3 (b) 4.7 (c) 7.5 (d) 9.3 (e) 9.7
Answer: (e)
11-118. What is the OH- ion concentration in a 0.200 M NaOAc solution? (HOAc: Ka = 1.8 x 10-5)
(a) 1.1 x 10-10 (b) 5.7 x 10-10 (c) 1.8 x 10-6
(d) 1.1 x 10-5 (e) 1.3 x 10-3
Answer: (d)
11-119. What is the H3O+ ion concentration in a 0.1 M NH3 solution? (Kb = 1.8 x 10-5)
(a) 7.5 x 10-12 (b) 3.0 x 10-10 (c) 1.8 x 10-6 (d) 1.3 x 10-3
(e) none of these
Answer: (a)
11-120. HA is a weak monoprotic acid with Ka = 2.4 x 10-6. What is [OH-] in a 0.300 M solution of the salt, NaA?
(a) 5.8 x 10-12 (b) 2.4 x 10-10 (c) 3.5 x 10-5
(d) 6.5 x 10-5 (e) 1.6 x 10-3
Answer: (c)
Buffers: General
11-121. Which of the following would make the best buffer?
(a) a mixture of HCl and NaCl
(b) a mixture of NaOAc and NH3
(c) a mixture of HOAc and NH4Cl
(d) a mixture of NaOAc and NH4Cl
(e) a mixture of NH3 and NH4Cl
Answer: (e)
11-122. Which of the following solutions would be an acidic buffer?
(a) 0.10 M HCl and 0.10 M NaOH
(b) 0.10 M HCl and 0.10 M NaCl
(c) 0.10 M HCO2H and 0.10 M NaHCO2
(d) 0.10 M NH3 and 0.10 M NH4Cl
(e) none of the above would be buffers.
Answer: (c)
11-123. When NH4Cl is added to an 0.10 M NH3 solution, the pH of this solution:
(a) increases (b) decreases (c) remains the same
(d) increases at first and then decreases.
(e) decreases at first and then increases.
Answer: (b)
11-124. When would the pH of a solution prepared by adding sodium formate to formic acid be equal to the pKa of formic acid, HCO2H?
(a) when [HCO2H] < [HCO2-]
(b) when [HCO2H] = [HCO2-]
(c) when [HCO2H] > [HCO2-]
(d) the pH of this buffer will never equal the pKa of formic acid.
Answer: (b)
11-125. How would you increase the buffering capacity of a buffer made by adding NaHCO2 to an aqueous solution of HCO2H?
(a) increase the concentration of HCO2H.
(b) increase the concentration of NaHCO2.
(c) increase the concentrations of both HCO2H and NaHCO2.
(d) increase the ratio of the concentration of HCO2H to NaHCO2.
(e) increase the ratio of the concentration of NaHCO2 to HCO2H.
Answer: (c)
11-126. Which of the following solutions would be the best buffer when a small amount of NaOH is added to the solution?
(a) a 1.0 L solution prepared by adding 0.10 mol of carbonic acid and 0.10 mol of sodium bicarbonate.
(b) a 1.0 L solution prepared by adding 0.20 mol of carbonic acid and 0.20 mol of sodium bicarbonate.
(c) a 1.0 L solution prepared by adding 0.10 mol of hydrochloric acid and 0.10 mol of sodium hydroxide.
(d) a 1.0 L solution prepared by adding 0.20 mol of hydrochloric acid and 0.20 mol of sodium hydroxide.
(e) all of these solutions would have the same buffer capacity.
Answer: (b)
11-127. Which of the following would be the best choice for preparing a pH = 8 buffer solution?
(a) A mixture of formic acid (Ka = 1.8 x 10-4) and the formate ion.
(b) A mixture of acetic acid (Ka = 1.8 x 10-5) and the acetate ion.
(c) A mixture of hypochlorous acid (Ka = 3.5 x 10-8) and the hypochlorite ion.
(d) A mixture of boric acid (Ka = 5.8 x 10-10) and its conjugate base.
(e) all of these mixtures would be equally good choices for making a pH = 8 buffer.
Answer: (c)
11-128. Which of the following solutions would have the smallest pH?
(HCO2H: Ka = 1.8 x 10-4; NH4+: Ka = 5.6 x 10-10)
(a) 0.10 M HCO2H (b) 0.10 M NaHCO2 (c) 0.10 M NH4+
(d) a solution that is 0.10 M in both HCO2H and NaHCO2
(e) a solution that is 0.10 M in both NH4+ and NH3.
Answer: (a)
11-129. Which of the following solutions will show the largest pH change when 10 mL of 1 M NaOH is added to it?
(a) 10 mL of 1 M NaOH (b) 100 mL of water
(c) 10 mL of 1 M NaNO3 (d) 10 mL of 0.01 M HOAc
(e) 100 mL of 0.1 M HOAc and 0.1 M NaOAc
Answer: (d)
[Note, 15% of the sample fell for answer: (b), instead of answer: (d)]
Buffers: Calculations
11-130. Calculate the initial concentration of HCO2Na you would have to add to 0.100 M HCO2H to make a buffer with a pH of 4.00. (HCO2H: Ka = 1.8 x 10-4)
Answer: 0.18 M
11-131. What is the pH of a solution that is simultaneously 0.10 M in both NH3 and the NH4+ ion? (NH4+: Ka = 5.6 x 10-10)
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH-(aq)
(a) 4.75 (b) 7.00 (c) 9.25
(d) the pH of this solution would be impossible to predict.
(e) none of the above.
Answer: (c)
11-132. What would be the effect of doubling the NH3 concentration in the solution described in the previous question?
(a) the pH would increase.
(b) the buffering capacity would increase.
(c) the NH4+ concentration would increase.
(d) all of the above statements are true.
(e) none of these statements are true
Answer: (a)
11-133. How much NaHCO2 would you have to add to 0.10 M HCO2H to get a buffer solution with a pH of 3.4? (HCO2H: Ka = 2 x 10-4)
(a) 0.010 M NaHCO2 (b) 0.050 M NaHCO2 (c) 0.10 M NaHCO2
(d) 0.20 M NaHCO2 (e) none of the above
Answer: (b)
11-134. How much NaOAc would have to be added to 1.00 L of 0.10 M HOAc to make a pH 3.00 buffer? (HOAc: Ka = 1.8 x 10-5)
Answer: 0.0018 M
11-135. How many moles of sodium acetate (NaOAc) must be added to 500 mL of 0.25 M acetic acid (HOAc) to give a pH 4.9 buffer solution? (HOAc: Ka = 1.8 x 10-5; assume no volume change.)
Answer: 0.18 mol
11-136. What is the [HOAc]/[OAc-] ratio in an acetic acid/sodium acetate buffer at pH = 4.9? (HOAc: Ka = 1.8 x 10-5)
(a) 0.31 (b) 0.70 (c) 1.4 (d) 2.4 (e) 4.90
Answer: (b)
11-137. Calculate the pH of a buffer prepared by mixing 0.10 mol of sodium formate and 0.05 mol of formic acid in 1.0 L of solution. (HCO2H: Ka = 1.8 x 10-4)
(a) 1.8 x 10-4 (b) 3.44 (c) 4.05 (d) 5.31 (e) none of the above
Answer: (c)
11-138. What is the pH of a solution that is 0.01 M in CH3NH2 and 0.1 M in CH3NH3+?
CH3NH2(aq) + H2O(l) ⇌ CH3NH3+(aq) + OH-(aq) Kb = 4.4 x 10-4
(a) 2.36 (b) 3.36 (c) 9.64 (d) 10.64 (e) 11.64
Answer: (c)
11-139. If equimolar amounts of NH3 and NH4Cl are dissolved in 1.00 L of water, what is the H3O+ ion concentration in this solution? (NH3: Kb = 1.8 x 10-5)
(a) 1.0 x 10-14 (b) 5.5 x 10-10 (c) 1.0 x 10-7 (d) 1.8 x 10-5
(e) it cannot be calculated from the information given.
Answer: (b)
Titration Curves
11-140. At what point in the following titration curve would the pH of the solution be equal to the pKa of the acid?

(a) 0 mL (b) 50 mL (c) 95 mL (d) 100 mL (e) 120 mL
Answer: (b)
Polyprotic Acids
11-141. Succinic acid (HO2CCH2CH2CO2H) is an intermediate in the the Kreb's cycle used to “burn” carbohydrates, proteins and lipids. The formula for succinic acid can be abbreviated as H2Sc. (H2Sc: Ka1 = 6.2 x 10-5; Ka2 = 2.3 x 10-6)
H2Sc(aq) + H2O(l) ⇌ H3O+(aq) + HSc-(aq) Ka1 = 6.9 x 10-5
HSc-(aq) + H2O(l) ⇌ H3O+(aq) + Sc2-(aq) Ka2 = 2.5 x 10-6
Which of the following assumptions may not be valid for solutions of succinic acid because the difference between Ka1 and Ka2 for H2Sc is very small.
(a) [H3O+] ≈ Kw/[OH-] (b) [H2Sc] < [Sc2-] (c) [HSc- ] ≈ [H3O+]
(d) [H2Sc] ≈ CH2Sc (e) None of these assumptions are legitimate for this solution.
Answer: (c)
11-142. What is the pH of an 0.10 M succinic acid solution if we assume that H2Sc is a weak acid that undergoes stepwise dissociation?
H2Sc(aq) + H2O(l) ⇌ H3O+(aq) + HSc-(aq) Ka1 = 6.9 x 10-5
HSc-(aq) + H2O(l) ⇌ H3O+(aq) + Sc2-(aq) Ka2 = 2.5 x 10-6
(a) 2.1 (b) 2.6 (c) 3.3 (d) 4.2 (e) 5.6
Answer: (b)
11-143. What fraction of the HSc- formed in the first step of the dissociation of succinic acid goes on to dissociate to form Sc2- ions in the second step, and what does this lead us to conclude?
(a) none of the HSc- ions dissociate further, and the stepwise dissociation assumption is legitimate.
(b) less than 1% of the HSc- ion dissociate to form Sc2- ions, and the stepwise dissociation assumption is legitimate.
(c) between 1 and 5% of the HSc- ions dissociate, and the stepwise dissociation assumption is legitimate.
(d) between 5 and 20% of the HSc- ions dissociate, and the stepwise dissociation assumption is legitimate.
(e) between 5 and 20% of the HSc- ions dissociate, and the stepwise dissociation assumption is not legitimate.
Answer: (b)
11-144. Which of the following equations accurately describes an 0.10 M H2Gly solution?
(a) [H3O+] ≈ [H2Gly] (b) [H3O+] > [H2Gly]
(c) [H3O+] < [HGly-] (d) [H3O+] ≈ [HGly-]
(e) [H3O+] > [HGly-]
Answer: (d)
11-145. For a weak diprotic acid such as glycine, it can be shown that:
(a) [Gly2-] ≈ CH2Gly (b) [Gly2-] ≈ Ka2 (c) [Gly2-] ≈ [HGly-]
(d) [Gly2-] > [HGly-] (e) [Gly2-] ≈ [H3O+]
Answer: (b)
11-146. Sulfurous acid (H2SO3) is an acid and sodium sulfite (Na2SO3) is a base. Which equation correctly describes the relationship between the equilibrium constants for these compounds?
(a) Ka1 x Kb2 = Kw (b) Ka1 x Ka2 = Kw (c) Ka2 x Kb2 = Kw
(d) Ka1 x Ka2 = 1 (e) Ka1 x Kb2 = 1
Answer: (a)
11-147. Use the relationship between Ka1 and Ka2 for H2SO3 and Kb1 and Kb2 for the SO32- ion to predict whether an 0.10 M solution of the HSO3- (bisulfite) ion would be acidic, basic or neutral. (H2SO3: Ka1 = 1.7 x 10-2, Ka2 = 6.4 x 10-8)
(a) the solution would be strongly acidic, pH < 2.
(b) the solution would be moderately acidic, pH≈ 4.
(c) the solution would be very close to neutral, pH ≈ 7.
(d) the solution would be slightly basic, pH ≈ 8.
(e) the solution would be strongly basic, pH > 12.
Answer: (b)
11-148. What is the pH of an 0.10 M H2CO3 solution? (H2CO3: Ka1 = 4.2 x 10-7, Ka2 = 4.8 x 10-11)
(a) 3.1 (b) 3.7 (c) 6.3 (d) 6.8 (e) none of the above
Answer: (b)
11-149. A diprotic acid, H2A, has the following dissociation constants: Ka1 = 2.1 x 10-7 and Ka2 = 4.3 x 10-13. The H3O+ ion concentration when this acid is dissolved in water is:
(a) roughly equal to the H2A concentration.
(b) much larger than the H2A concentration.
(c) much larger than the HA- concentration.
(d) approximately equal to the HA- concentration.
(e) much less than the HA- concentration.
Answer: (d)
11-150. For a weak diprotic acid, H2A, for which Ka1 = 2.1 x 10-7 and Ka2 = 4.3 x 10-13 the A2- ion concentration at equilibrium will be:
(a) approximately equal to the initial concentration of H2A.
(b) roughly equal to Ka2.
(c) roughly equal to the HA- concentration.
(d) much larger than the HA- concentration.
(e) approximately equal to the H3O+ concentration
Answer: (b)
Polyprotic Bases
11-151. Which of the following equations can be used to calculate Kb1 and Kb2 for Na2Gly from Ka1 and Ka2 for H2Gly?
(a) Kb1 = Kw x Ka1 and Kb2 = Kw x Ka2
(b) Kb1 = Kw/Ka1 and Kb2 = Kw/Ka2
(c) Kb1 = Ka1/Kw and Kb2 = Ka1/Kw
(d) Kb1 = Kw x Ka2 and Kb2 = Kw x Ka1
(e) Kb1 = Kw/Ka2 and Kb2 = Kw/Ka1
Answer: (e)
11-152. For H2CO3, Ka2 = 4.7 x 10-11. Which of the following numbers is closest to the pH of a 0.200 M Na2CO3 solution? Hint: Only the first step in the hydrolysis of the CO32- ion need be considered in this calculation.
(a) 5.9 x 10-3 (b) 2.2 (c) 2.48 (d) 11.6 (e) 11.8
Answer: (e)
Polyprotic Acids and Bases
Use the following information to answer questions 153-155.
H2Se: Ka1 = 1.7 x 10-4, Ka2 = 1.0 x 10-10
11-153. In a 0.10 M solution of H2Se, the Se2- concentration is:
(a) [Se2-] = 0.10 x Ka2 = 1.0 x 10-11 (b) [Se2-] = Ka2 = 1.0 x 10-10
(c) [Se2-] = (0.10 x Ka2)1/2 = 3.2 x 10-6 (d) [Se2-] = (0.10 x Ka1)1/2 = 4.1 x 10-3
(e) none of the above
Answer: (b)
11-154. If 0.20 moles per liter of sodium selenide is dissolved in water, the base-ionization constant for the Se2- ion would be:
(a) Kb1 = Ka1 = 1.7 x 10-4 (b) Kb1 = Ka2 = 1.0 x 10-10
(c) Kb1 = Kw/Ka1 = 5.9 x 10-11 (d) Kb1 = Kw/Ka2 = 1.0 x 10-4
(e) none of the above
Answer: (d)
11-155. If sodium selenide is added to pure water, we would expect the pH to:
(a) increase (b) decrease (c) remain the same
(d) change in a direction that can't be predicted from this information.
Answer: (a)
Polyprotic Acids or Bases
11-156. KHS can act as a weak acid or a weak base.
HS-(aq) + H2O(l) ⇌ S2-(aq) + H3O+(aq) Ka2 = 1.3 x 10-13
HS-(aq) + H2O(l) ⇌ H2S(aq) + OH-(aq) Kb2 = 1.0 x 10-7
Which of the following statements is correct?
(a) a solution of 0.1 M K2S and 0.1 M KHS will be acidic.
(b) a solution of 0.01 M H2S will have a pH = 2.
(c) a solution of 0.1 M KHS will be weakly basic.
(d) H2S is over 90% ionized in water solution.
(e) the S2- ion concentration in a 0.1 M KHS solution does not depend on pH.
Answer: (c)
11-157. Hydrogen sulfide (H2S) is an acid, and sodium sulfide (Na2S) is a base.
(H2S: Ka1 = 1.0 x 10-7, Ka2 = 1.3 x 10-13)
Which of the following statements concerning NaSH is correct?
(a) NaSH is an acid because Ka1 for H2S is much larger than Ka2.
(b) NaSH is an acid because Ka1 for H2S is smaller than Kb1 for Na2S.
(c) NaSH is a base because Kb1 for Na2S is much larger than Kb2.
(d) NaSH is a base because Kb2 for Na2S is larger than Ka2 for H2S.
(e) it is impossible to tell whether NaSH is an acid or a base from these data.
Answer: (d)
11-158. H2Gly is an acid and Na2Gly is a base. On the basis of the values of Ka1 and Ka2 for H2Gly and the values of Kb1 and Kb2 for Na2Gly, would an 0.10 M NaHGly solution be acidic, basic, or neutral? (H2Gly: Ka1 = 4.5 x 10-3, Ka2 = 2.5 x 10-10)
(a) slightly acidic (b) slightly basic (c) neutral (pH = 7)
(d) there is no way to predict whether NaHGly is an acid or base.
Answer: (a)