
CHAPTER 2:
THE MOLE: THE LINK BETWEEN THE
MACROSCOPIC AND THE ATOMIC WORLD OF CHEMISTRY
Atomic/Molecular Weight
2-1. What is the atomic weight of an element for which a single atom weighs 3.95 x 10-22 g? (a) 6.56 x 10-46 (b) 1.31 x 10-45 (c) 3.95 x 10-22 (d) 238 (e) 476
Answer: (d)
2-2. Suppose that a superheavy element with atomic number 120 is found on an expedition to Io, one of the moon's of Jupiter. Due to its scarcity, only 2 x 1014 atoms could be isolated. If this sample weighed 100 ng, what was the element's atomic mass in amu's? (a) less than 1 amu (b) between 1 amu and 100 amu
(c) between 100 amu and 250 amu (d) between 250 amu and 400 amu
(e) more than 500 amu
Answer: (d)
2-3. What is the molecular weight of aspirin, C9H8O4? (AW: H = 1.00; C = 12.0; O = 16.0 amu)
(a) less than 50 amu (b) between 50 and 80 amu (c) between 80 and 120 amu
(d) between 120 and 160 amu (e) more than 160 amu
Answer: (e)
2-4. Cocaine is extracted from the leaves of plants of the species E. coca, whereas chocolate is extracted from the seeds of the species E. cacao. (Never confuse one with the other!) Calculate the molecular weight of cocaine, assuming a molecular formula of C17H21NO4. (AW: H = 1.0, C = 12.0, N = 14.0, O = 16.0 amu)
(a) between 0 and 100 g/mol (b) between 100 and 250 g/mol
(c) between 250 and 300 g/mol (d) between 300 and 350 g/mol
(e) more than 350 g/mol
Answer: (d)
2-5. What is the mass of a molecule of sucrose (cane sugar) that has the formula: C12H22O11? (H = 1.0; C = 12.0; O = 16.0 amu)
(a) less than 100 amu (b) between 100 and 150 amu
(c) between 150 and 250 amu (d) between 250 and 350 amu
(e) more than 350 amu
Answer: (d)
2-6. Calculate the molecular weight of the oxygen-carrier protein hemoglobin if this protein is 0.335% Fe by weight and each protein molecule contains four iron atoms. (AW: Fe = 55.85 amu)
(a) less than 1000 grams per mole
(b) between 1000 and 10,000 grams per mole
(c) between 10,000 and 50,000 grams per mole
(d) between 50,000 and 100,000 grams per mole
(e) more than 100,000 grams per mole
Answer: (d)
2-7. What is the mass of a molecule of CO2?
(a) 4.6 x 10-23 g (b) 7.3 x 10-23 g (c) 28 g (d) 44 g
(e) none of the above
Answer: (b)
2-8.The drug for which more prescriptions – and more bad jokes – were written than any drug released recently has the scientific name Sildenafil citrate and the trade name Viagra™. The structure of Sildenafil is shown below.

When this line structure is translated into a chemical formula we get a molecular formula of C22H32N6O4S. Calculate the molecular weight of this compound. (AW: H = 1.008, C = 12.01, N = 14.00, O = 16.00, S = 32.06 g/mol)
(a) between 100 and 200 g/mol (b) between 200 and 300 g/mol
(c) between 300 and 400 g/mol (d) between 400 and 500 g/mol
(e) more than 500 g/mol
Answer: (d)
2-9. Let’s consider another “miracle drug” with the scientific name orlistat, which is marketed by Roche Pharmaceuticals as Xenical®. The formal chemical name for Xenical® is (S)-2-formylamino-4-methyl-pentanoic acid (S)-1-[[(2S, 3S)-3-hexyl-4-oxo-2-oxetanyl] methyl]-dodecyl ester. Its structure is shown below.
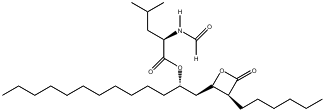
The molecular formula for this wonder diet drug that is supposed to block the uptake of fat is C29H35NO5. Calculate the molecular weight of this compound. (AW: H = 1.008, C = 12.01, N = 14.00, O = 16.00 g/mol)
(a) between 100 and 200 g/mol (b) between 200 and 300 g/mol
(c) between 300 and 400 g/mol (d) between 400 and 500 g/mol
(e) more than 500 g/mol
Answer: (d)
2-10. If you eat too much fat while taking Xenical®, you’re going to be very, very “uncomfortable.” You can avoid this problem, of course, by taking the other new diet drug, Sibutramine hydrochloride monohydrate, which is marketed by a subsidiary of BASF as Meridia®. Meridia® is a neurotransmitter that inhibits the uptake of other neurotransmitters, such as dopamine, norepinephrine, and serotonin. It therefore tends to depress one’s appetite and is being studied as a possible treatment for depression. Elemental analysis suggests that this compound is 72.96% C, 9.36% H, 12.67% Cl, and 5.01% N by weight. What is the total number of C, H, N and Cl atoms in the molecular formula of this compound if the molecular weight is 279.85 g/mol?
(a) 10 or less (b) between 11 and 20 (c) between 21 and 30
(d) between 31 and 40 (e) between 41 and 50
Answer: (e)
2-11. ADM (Archer Daniels Midland) the self-proclaimed “Supermarket to the World” has been in trouble for fixing the price of lysine, an amino acid that is often deficient in vegetarian diets. Lysine is 19.16% N by weight and each molecule contains two nitrogen atoms. Calculate the molecular weight of lysine. (AW: N = 14.01 g/mol)
(a) less than 100 g/mol (b) between 100 and 150 g/mol
(c) between 150 and 250 g/mol (d) between 250 and 350 g/mol
(e) more than 350 g/mol
Answer: (b)
The Mole Concept
2-12. Calculate the number of carbon atoms in a mole of sucrose, C12H22O11. (AW: H = 1.008, C = 12.01, O = 16.00 amu)
(a) 12 (b) 6.02 x 1023 (c) 6.62 x 1024 (d) 7.22 x 1024 (e) 1.3 x 1025
Answer: (d)
2-13. Calculate the number of chlorine atoms in 10.4 grams of chloroform, CHCl3. (AW: H = 1.008, C = 12.01, Cl = 35.45 amu)
(a) 5.25 x 1022 (b) 1.05 x 1023 (c) 1.57 x 1023
(d) 2.10 x 1023 (e) 2.07 x 1025
Answer: (c)
2-14. Sodium tripolyphosphate, Na5P3O10, is added to detergents to increase their cleaning power. Calculate the number of phosphorus atoms in 0.325 moles of this compound.
(a) 6.52 x 1022 (b) 1.96 x 1023 (c) 5.87 x 1023 (d) 6.02 x 1023
(e) None of the above
Answer: (c)
2-15. Calculate the number of electrons in 34.0 g of OH- ions.
(a) 8 (b) 10 (c) 20 (d) 1.2 x 1024 (e) 1.20 x 1025
Answer: (e)
2-16. Which of the following contains the largest number of hydrogen atoms?
(a) 1 mol of H2O (b) 0.5 mol of NH3
(c) 0.20 mol of CH4 (d) 0.20 mol of C6H12O6
(e) 0.01 mol of H3PO4
Answer: (a)
2-17. Which of the following contains the largest number of carbon atoms?
(a) 0.10 moles of acetic acid, CH3CO2H
(b) 0.25 moles of carbon dioxide, CO2
(c) 0.050 moles of glucose, C6H12O6
(d) 0.010 moles of sucrose, C12H22O11
(e) all of the above have the same number of carbon atoms
Answer: (b)
2-18. Which pair of samples contains the same number of hydrogen atoms:
(a) one mole of NH3 and one mole of N2H4
(b) two moles of NH3 and one mole of N2H4
(c) one mole of NH3 and two moles of N2H4
(d) two moles of NH3 and three moles of N2H4
(e) four moles of NH3 and three moles of N2H4
Answer: (e)
2-19. There are 16 ounces in a lb and 2.20 lbs in a kg. What would be the value of Avogadro's number if this constant was defined as the number of 12C atoms in 12 ounces of 12C?
(a) 0.171 x 1023 (b) 6.02 x 1023 (c) 28.4 x 1023
(d) 171 x 1023 (e) 2050 x 1023
Answer: (d)
2-20. How many platinum atoms does 1.00 g of pure platinum contain? (AW: Pt = 195.08 g/mol)
(a) 195 (b) 3.09 x 1021 (c) 6.17 x 1021 (d) 1.95 x 1023 (e) 6.02 x 1023
Answer: (b)
2-21. If the atomic weight of platinum is 195.08 g/mol, what is the mass of a single platinum atom?
(a) 1.62 x 10-22 g (b) 3.24 x 10-22 g (c) 5.13 x 10-3 g
(d) 195.08 g (e) none of the above
Answer: (b)
2-22. In lecture, I fired a large brass cannon that was fueled by a mixture of 2,2,5-trimethyl-pentane (or iso-octane) and oxygen. The molecular formula of iso-octane is C8H18. It burns in the presence of oxygen to form a mixture of CO2 and H2O. What is the ratio of moles of water to moles of carbon dioxide produced in this reaction?
(a) 1:1 (b) 2:1 (c) 9:4 (d) 9:8 (e) none of the above
Answer: (d)
2-23. How many grams of CO2 gas are produced from the combustion of 10.0 grams of iso-octane? (AW: H = 1.008, C = 12.01, O = 16.00 g/mol)
(a) 1.58 g (b) 3.85 g (c) 14.19 g (d) 30.82 g (e) 35.02 g
Answer: (d)
Empirical/Molecular Formulas
2-24. Apatite is a mineral that is found in tooth enamel. When fluoride toothpastes are used, this mineral is converted to fluoroapatite, which is much harder, and therefore more resistant to decay. What is the percent by weight of fluorine in fluoroapatite, Ca5(PO4)3F? (AW: O = 16.00, F = 19.00, P = 30.97, Ca = 40.08 amu)
(a) between 0 and 0.1% (b) between 0.1% and 1%
(c) between 1% and 3% (d) between 3% and 5% (e) more than 5%
Answer: (d)
2-25. Calculate the number of carbon atoms in the empirical formula of nicotine if this compound is 74.0% C, 8.7% H, and 17.3% N by weight. (AW: H = 1.008; C = 12.01; N = 14.01 amu)
(a) 3 (b) 4 (c) 5 (d) 7 (e) 10
Answer: (c)
2-26. Glucose (blood sugar), acetic acid, and formaldehyde are all 40.0% C, 6.76% H, and 53.3% O by weight. Calculate the total number of carbon, hydrogen, and oxygen atoms in the empirical formula for these compounds. (AW: H = 1.01, C = 12.0, O = 16.0 amu)
(a) 3 (b) 4 (c) 5 (d) 6 (e) 9
Answer: (b)
2-27. A compound that is 43.64% P and 56.36% O by weight has a molecular weight of 283.88 g/mol. What is the molecular formula of this compound? (A.W.: O = 16.00, P = 30.97 amu)
(a) PO4 (b) P2O3 (c) P2O5 (d) P4O10 (e) none of the above
Answer: (d)
2-28. A mixed oxide of potassium and vanadium is 28.3% by weight potassium and 37.0% by weight vanadium. What is the empirical formula? (AW: O = 16.00; K = 39.10; V = 50.94 amu)
(a) KV (b) KVO (c) K2V3O (d) K3V3O (e) KVO3
Answer: (e)
2-29. The chief ore of manganese is an oxide known as pyrolusite, which is 36.8% O and 63.2% Mn by weight. Which of the following is the correct empirical formula for pyrolusite?
(a) MnO (b) MnO2 (c) Mn2O3 (d) MnO3 (e) Mn2O7
Answer: (b)
2-30. Nitrogen combines with oxygen to form a variety of compounds. One of these compounds is called nitrous oxide or “laughing gas.” What is the formula of nitrous oxide if this compound is 63.65% N and 36.35% O by weight?
(a) N2O (b) NO (c) NO2 (d) N2O3 (e) N2O5
Answer: (a)
2-31. What is the atomic weight of a metal that forms an oxide, M2O3, that is 17.29% oxygen by weight?
(a) 38.3 (b) 57.4 (c) 76.6 (d) 115 (e) 229 g/mol
Answer: (d)
2-32. CISPLATIN is a commercial antitumor drug that contains 65.0% Pt, 23.7% Cl, 9.3% N and 2.0% H by weight. What is the ratio of H to Pt atoms in the empirical formula of this compound? (H = 1.0; N = 14.0; Cl = 35.5; Pt = 195.1 amu)
(a) 1:1 (b) 2:1 (c) 3:1 (d) 4:1 (e) 6:1
Answer: (e)
2-33. What is the empirical formula of a compound of nitrogen and oxygen if a sample of the compound contains 0.483 grams of nitrogen and 1.104 grams of oxygen. (AW: N = 14.0, O = 16.0 amu)
(a) N2O (b) NO (c) NO2 (d) N2O3 (e) N2O5
Answer: (c)
2-34. What is the atomic weight of the element that forms a fluoride, M2F4, that is 73.1% fluorine by weight? (AW: F = 19.0 amu)
(a) 14.0 amu (b) 16.0 amu (c) 28.0 amu (d) 32.0 amu (e) none of these
Answer: (a)
2-35. What is the empirical formula of a compound that contains C, H, and O if the compound is 40.00% C and 6.71% H by weight?
(a) CHO (b) CH2O (c) CH3O (d) CH4O (e) none of the above
Answer: (b)
2-36. Calculate the empirical formula of napthalene if this compound is 93.71% carbon and 6.29% hydrogen by weight. (AW: H = 1.008; C = 12.01 amu)
(a) CH (b) CH2 (c) C3H2 (d) C4H5 (e) C5H4
Answer: (e)
2-37. The molecular weight of napthalene is 128.17 grams per mole. Use the results of the previous question to calculate the total number of atoms in a napthalene molecule.
(a) 9 (b) 12 (c) 18 (d) 27 (e) 38
Answer: (c)
2-38. The term “carbohydrate” once meant a compound with the empirical formula CH2O. What is the molecular formula of glucose (blood sugar) if the molecular weight of this carbohydrate is 180 g/mol? (H = 1.0; C = 12.0; O = 16.0 amu)
(a) CH2O (b) C4H8O4 (c) C6H12O6 (d) C12H22O11 (e) none of the above
Answer: (c)
2-39. The ribonucleic acids (RNA) that are so important in the biosynthesis of proteins all contain the sugar D-ribose, which contains three elements: C, H, and O. Elemental analysis suggests that D-ribose is 40.00% C and 6.71% H by weight. What is the empirical formula of this compound? (AW: H = 1.008, C = 12.01, O = 16.00 amu)
(a) CHO (b) CH2O (c) CH3O (d) CH3O2 (e) none of these
Answer: (b)
2-40. A 1.854 g sample of D-ribose contains 0.01236 moles of this sugar. What is the ratio of the molecular formula to the empirical formula of this compound?
(a) 1 (b) 2 (c) 3 (d) 5 (e) none of these
Answer: (d)
2-41. Caffeine is a central nervous system stimulant found in coffee, tea, and cola nuts. Calculate the molecular formula if caffeine is 49.48% C, 5.19% H, 28.85% N and 16.48% O by weight and the molecular weight is 194.2 g/mol.
Answer: C8H10N4O2
2-42. Aspartame is an artificial sweetener 160 times sweeter than cane sugar, which is 57.14% C, 6.16% H, 9.52% N and 27.18% 0 by weight. Calculate the molecular formula of this compound if the molecular weight is 294.30 g/mol.
Answer: C14H18N2O5
2-43. Halothane is an anaesthetic that is 12.17% C, 0.51% H, 40.48% Br, 17.96% Cl and 28.87% F by weight. Calculate the molecular formula of this compound if each molecule contains one hydrogen atom.
Answer: C2HBrClF3
2-44. In small quantities, the nicotine in tobacco is addictive. In large quantities, it is a deadly poison. Calculate the molecular formula of nicotine, CxHyNz, if the molecular weight is 162.2 g/mol and 0.438 grams of this compound burn to form 1.188 grams of CO2 and 0.341 grams of water.
Answer: C10H14N2
2-45. A compound frequently used as a high-temperature lubricant has the formula MS2, where M represents some metallic element. If is compound is 40.06% sulfur by mass, what is the identity of the metal M?
(a) K (AW = 39.9 amu) (b) Fe (AW = 55.85 amu) (c) Cu (AW = 63.55 amu) (d) Mo (AW = 95.95 amu) (e) Pb (AW = 207.2 amu)
Answer: (c)
2-46. In 1763, the Reverend Edmund Stone suggested chewing the bark of the English willow (Salix abla) to treat a fever because it grows in moist regions where one was likely to catch a fever, and “... many natural maladies carry their cures along with them, or their remedies lie not far from their causes.” It took 50 years to isolate the active ingredient in the bark, which was named salicylic acid. By the end of the 19th century, salicylic acid was used to treat rheumatic fever, gout and arthritis. Many patients complained of chronic stomach irritation, however. Because his father was one of these patients, Felix Hoffman searched for a less acidic derivative of salicylic acid. In 1898, Hoffman reported that the acetyl ester of salicylic acid was both more effective and easier to tolerate than the parent compound. He named the compound aspirin, taking the prefix a- from the name of the acetyl group and spirin from the German name of the parent compound spirsure. Aspirin is 60.00% C, 4.48% H, and 35.5% O by weight. What is the total number of C, H and O atoms in the empirical formula of this compound?
(a) Less than 15 (b) Between 15 and 20 (c) Between 20 and 25
(d) Between 25 and 35 (e) More than 35
Answer: (b)
2-47. On March 20, 1995, terrorists released an organophosphate nerve gas known as “Sarin” at several points in the Tokyo subway system, killing 11 and injuring more than 5,500 people. Sarin was concealed in lunch boxes and soft-drink containers and placed on subway train floors. It was released as terrorists punctured the containers with umbrellas before leaving the trains. Elemental analysis of Sarin gives the following result: 34.29% C, 7.19% H, 22.84% O, 13.56% F, 22.11% P. How many carbon atoms does the empirical formula of this compound contain? (AW: H = 1.008, C = 12.01, O = 16.00, F = 19.00, P = 30.97 g/mol)
(a) 2 or less (b) 3 to 6 (c) 7 to 10 (d) 11 to 15 (e) more than 15
Answer: (b)
2-48. The molecular weight of Sarin (or methylphosphonofluoridic acid 1-methyl ethyl ester) is the same as the empirical weight of this compound. What is the molecular weight of Sarin in grams per mole?
(a) less than 100 grams/mole (b) between 100 and 150 grams/mole
(c) between 150 and 200 grams/mole (d) between 200 and 250 grams/mole
(e) more than 250 grams/mole
2-49. One of the concerns during the war against Iraq several years ago was fear of chemical warfare agents, such as the nerve agent VX. VX is the most dangerous material ever synthesized; a single drop of VX on the skin will kill an individual within hours. More than 2 million pounds of VX were made in Southern Indiana by 1968. They are still there, stored in 1600 canisters, packed three high. The canisters were welded together several years ago because of fear of exposure to VX if a tornado struck the storage facility. Elemental analysis of VX gives the following result: 49.41% C, 9.80% H, 5.24% N, 11.97% O, 11.58% P, and 11.99% S. How many carbon atoms does the empirical formula for this compound contain? (Hint: Carry out the calculation to at least three significant figures.)
(a) 3 or less (b) 4 to 7 (c) 8 to 11 (d) 12 to 15 (e) more than 15
Answer: (c)
2-50. Assume that the empirical formula of VX is the same as the molecular formula. What is the molecular weight of VX?
(a) less than 150 grams/mole (b) between 150 and 200 grams/mole
(c) between 200 and 250 grams/mole (d) between 250 and 300 grams/mole
(e) more than 300 grams/mole
Answer: (d)
Balancing Equations
2-51. The balanced equation for the decomposition of ammonium dichromate
A (NH4)2Cr2O7(s) ➝ B Cr2O3(s) + C N2(g) + D H2O(g)
has which of the following sets of coefficients?
(a) A = 2, B = 2, C = 2, D = 4 (b) A = 1, B = 1, C = 1, D = 4
(c) A = 1, B = 1, C = 1, D = 2 (d) A = 1, B = 1, C = 2, D = 2
(e) A = B = C = D = 1
Answer: (b)
2-52. Calculate the sum of the coefficients in the balanced chemical equation for the following reaction: A H2S(g) + B O2(g) ➝ C SO2(g) + D H2O(g)
(a) 6 (b) 8 (c) 9 (d) 11 (e) 17
Answer: (c)
2-53. What is the sum of the coefficients when the following chemical equation is balanced?
A Ca3(PO4)2(s) + B C(s) ➝ C Ca3P2(s) +D CO(g)
(a) 6 (b) 12 (c) 18 (d) 20 (e) none of the above
Answer: (c)
Stoichiometry
2-54. Thermal decomposition of an unknown carbonate lead to a 35.1% weight loss. The unknown was which of the following compounds.
(a) Li2CO3 (b) MgCO3 (c) CaCO3 (d) ZnCO3 (e) BaCO3
Answer: (d)
2-55. Calcium carbonate decomposes on heating to form calcium oxide and carbon dioxide:
CaCO3(s) ➝ CaO(s) + CO2(g).
Which of the following would cause the weight loss during the decomposition of a sample of CaCO3 to appear too large?
(a) The CaCO3 contained some CaO before it was heated.
(b) The crucible was wet when it was weighed before the CaCO3 was heated.
(c) The crucible was heated for too short a time.
(d) The student read the last weight as 40.3047 g instead of the actual value of 30.3047 g.
(e) All of these would cause the weight loss to appear too large.
Answer: (b)
2-56. A crucible and sample of CaCO3, weighing 42.670 g, was heated until red hot to decompose the sample.
CaCO3(s) ➝ CaO(s) + CO2(g)
The crucible weighed 35.351 g. What is the theoretical weight of the crucible and residue after the decomposition is complete?
(a) 3.219 g (b) 4.100 g (c) 38.570 g (d) 39.451 g (e) 49.989 g
Answer: (d)
2-57. Nitrogen reacts with hydrogen to form ammonia
N2(g) + 3 H2(g) ➝ 2 NH3(g)
which burns in the presence of oxygen to form nitrogen oxide,
4 NH3(g) + 5 O2(g) ➝ 4 NO(g) + 6 H2O(g)
which reacts with excess oxygen to form nitrogen dioxide.
2 NO(g) + O2(g) ➝ 2 NO2(g)
How much nitrogen would we have to start with to make 10 moles of nitrogen dioxide?
(a) 2.5 mol (b) 5 mol (c) 10 mol (c) 15 mol (d) 20 mol
Answer: (b)
2-58. How many grams of hydrogen peroxide, H2O2, must decompose by the following reaction to produce 0.400 moles of O2? (AW: H = 1.008 amu; O = 16.00 amu)
2 H2O2(aq) ➝ 2 H2O(l) + O2(g)
(a) less than 25 grams (b) between 25 and 40 grams (c) between 40 and 60 grams
(d) between 60 and 80 grams (e) more than 80 grams
Answer: (b)
2-59. Which of the following nitrogen-containing compounds would supply the most nitrogen per gram of fertilizer?
(a) iron azide, Fe(N3)2, MW = 140 g/mol
(b) sodium azide, NaN3, MW = 83 g/mol
(c) potassium nitrite, KNO2, MW = 85.1 g/mol
(d) potassium azide, KN3, MW = 81.1 g/mol
(e) there is not enough information to answer this problem
Answer: (a)
2-60. How many oxygen molecules are required to consume 15.5 grams of phosphorus when P4 is burned in oxygen to make tetraphosphorus decaoxide?
(a) 2.50 (b) 5.00 (c) 10.0 (d) 3.76 x 1023 (e) 3.01 x 1024
Answer: (d)
2-61. The chemical formula of ethanol is C2H6O. It burns in excess oxygen to form CO2 and H2O. How many grams of H2O are produced from the combustion of 25.0 g of ethanol? (AW: H = 1.01; C = 12.0; O = 16.0 amu)
(a) 9.78 g (b) 18.0 g (c) 25.0 g (d) 29.3 g (e) 54.1 g
Answer: (d)
2-62. PF3 reacts with XeF4 to give PF5. In theory, how many moles of PF5 can be produced from 100.0 g of PF3 and 50.0 g of XeF4?
2 PF3(g) + XeF4(s) ➝ 2 PF5(g) + Xe(g)
(AW: F = 19.00; P = 30.97; Xe = 131.3 amu)
(a) 0.121 mol (b) 0.241 mol (c) 0.482 mol (d) 1.14 mol (e) 2.28 mol
Answer: (c)
2-63. The portable stoves campers use for cooking burn propane, C3H8.
A C3H8(g) + B O2(g) ➝ C CO2(g) + D H2O(g)
What weight of propane would have to be burned to form 7.26 g of CO2 (AW: H = 1.0; C = 12.0; O = 16.0 amu)
(a) 2.42 g (b) 4.84 g (c) 7.26 g (d) 21.8 g (e) none of the above
Answer: (a)
2-64. Calculate the weight of O2 needed to burn 10.0 grams of hydrogen sulfide, H2S. (AW: H = 1.008, O = 16.00, S = 32.06 amu)
A H2S + B O2 ➝ C SO2 + D H2O
(a) 4.70 g (b) 6.26 (c) 7.04 g (d) 9.39 g (e) 14.1 g
Answer: (e)
2-65. What would happen to the potential yield of sulfur dioxide in the previous question if the amount of oxygen was doubled?
(a) it would decrease by a factor of 2. (b) it would decrease by a factor of l.5.
(c) it would remain constant. (d) it would increase by a factor of 1.5.
(e) it would increase by a factor of 2.
Answer: (c)
2-66. A sample of copper(II) sulfate, CuSO4, that weighs 2.47 grams picks up water from the atmosphere to form a hydrate with the formula CuSO4 ∙ x H2O. If the sample weighs 3.86 grams after it picks up water, what is the value of x? (AW, H = 1.01, O = 16.0, S = 32.0, Cu = 63.5 amu)
(a) 2 (b) 2.5 (c) 3 (d) 4 (e) 5
Answer: (e)
2-67. Nitrogen reacts with red-hot magnesium to form magnesium nitride,
3 Mg(s) + N2(g) ➝ Mg3N2(s)
which reacts with water to form magnesium hydroxide and ammonia,
Mg3N2(s) + 6 H2O(l) ➝ 3 Mg(OH)2(aq) + 2 NH3(aq)
How many grams of magnesium would you have to start with to prepare 15.0 grams of ammonia? (AW: H = 1.01, N = 14.0, O = 16.0, Mg = 24.3 amu)
(a) 13.3 g (b) 15.0 g (c) 20.0 g (d) 32.2 g (e) none of the above
Answer: (d)
2-68. There are many different kinds of “sugars.” Glucose, or “blood sugar,” and fructose, or “fruit sugar,” have the formula C6H12O6. Sucrose, or “cane sugar,” has the formula C12H22O11. What weight of carbon dioxide can be produced from 10.0 grams of sucrose and 10.0 grams of oxygen? (AW: H = 1.008, C = 12.01, O = 16.00 g/mol)
C12H22O11(s) + 12 O2(g) ➝ 12 CO2(g) + 11 H2O(g)
(a) Less than 5 grams (b) Between 5 and 10 grams
(c) Between 10 and 15 grams (d) Between 15 and 20 grams
(e) More than 20 grams.
Limiting Reagents
Questions 69-70 refer to the following reaction:
2 NO(g) + O2(g) ➝ 2 NO2(g)
2-69. What is the limiting reagent when 10.0 g of NO react with 10.0 g of O2 to form NO2? (AW: N = 14.0, O = 16.0 amu/atom)
(a) NO (b) O2 (c) NO2 (d) none of these
(e) there aren't enough data to answer the question.
Answer: (a)
2-70. How many moles of the limiting reagent are present in the previous problem?
(a) 0.313 (b) 0.333 (c) 0.435 (d) 0.625 (e) none of these
Answer: (b)
2-71. J. J. Berzelius found that 11.56 grams of lead sulfide, PbS, were formed when 10.0 grams of lead react with 1.56 grams of sulfur, when 10.0 grams of lead react with 3.00 grams of sulfur, and when 18.0 grams of lead react with 1.56 grams of sulfur. Explain his observations in terms of the concept of limiting reagent.
2-72. How many grams of HCl are produced when 10.0 g of Cl2 and 1.00 g of H2 react? (A.W.: H = 1.008, Cl = 35.45 amu)
H2(g) + Cl2(g) ➝ 2 HCl(g)
(a) 5.14 g (b) 9.04 g (c) 10.3 g (d) 11.0 g (e) 18.1 g
Answer: (c)
2-73. What would happen to the amount of HCl produced in the previous question if the amount of H2 is doubled?
(a) it would decrease by more than a factor of two.
(b) it would decrease by a factor of two.
(c) it would remain the same.
(d) it would increase by a factor of two.
(e) it would increase by more than a factor of two.
Answer: (c)
2-74. How many grams of MgO can be produced by burning 10.0 grams of Mg in the presence of 10.0 grams of O2?
(a) 10.0 g (b) 12.6 g (c) 16.6 g (d) 20.0 g (e) 25.2 g
Answer: (c)
2-75. When glucose reacts with oxygen in living systems, carbon dioxide and water are produced, and a great deal of energy is liberated.
C6H12O6(s) + 6 O2(g) ➝ 6 CO2(g) + 6 H2O(g)
What weight of carbon dioxide can be produced from the reaction of 10.0 grams of glucose with 10.0 grams of oxygen? (AW, H = 1.01, C = 12.0, O = 16.0 amu)
(a) 2.29 g (b) 2.44 g (c) 13.8 g (d) 14.7 g (e) none of these
Answer: (d)
Solutions
2-76. “Muriatic acid” is sold in many hardware stores for cleaning bricks and tile. What is the molarity of this solution if 125 mL of the solution contains 27.4 grams of HCl? (H = 1.0; Cl = 35.5 amu)
(a) 0.0938 M (b) 0.751 M (c) 3.43 M (d) 6.01 M (e) 219 M
Answer: (d)
2-77. How many grams of baking soda, NaHCO3, would you need to neutralize 15 mL of muriatic acid? (H = 1.0; C = 12.0; 0 = 16.0; Na = 23.0; Cl = 35.5 amu)
(a) less than 1 g (b) between 1 and 5 grams (c) between 5 and 10 grams
(d) between 10 and 20 grams (e) more than 20 grams
Answer: (c)
2-78. Calculate the concentration of a solution prepared by dissolving 10.0 grams of NaNO3 in enough water to give 250.0 mL of solution. (AW: N = 14.0; O = 16.0; Na = 23.0 amu)
(a) 0.0294 M (b) 0.118 M (c) 0.471 M (d) 2.13 M (e) 8.50 M
Answer: (c)
2-79. What is the molarity of concentrated phosphoric acid if this solution is 85.5% H3PO4 by weight and it has a density of 1.70 g/mL? (AW: H = 1.0, 0 = 16.0, P = 31.0 amu)
(a) less than 5 M (b) 14.8 M (c) 17.3 M (d) 20.3 M (e) none of the above
Answer: (b)
2-80. Describe in detail the steps you would take in the lab to prepare 125 mL of 0.745 M oxalic acid, H2C2O4 ∙ 2 H2O. Describe the glassware you would need, the chemicals, the amounts of each chemical, and the sequence of steps you would take.
2-81. How many mL of 15 M NH3 would you need to make 15.0 mL of a 3.6 M NH3 solution? (a) 0.036 mL (b) 0.63 mL (c) 3.6 mL (d) 8.1 mL (e) 360 mL
Answer: (c)
2-82. Calculate the volume of 0.0100 M sulfuric acid (H2SO4) that would be needed to neutralize 10.00 mL of an 0.0100 M aqueous ammonia (NH3) solution:
H2SO4(aq) + 2 NH3(aq) ➝ (NH4)2SO4(aq)
(a) 2.50 mL (b) 5.00 mL (c) 10.0 mL (d) 15.0 mL (e) 20.0 mL
Answer: (b)
2-83. What is the molarity of an oxalic acid solution if it takes 37.55 mL of 0.1245 M sodium hydroxide to titrate 10.00 mL of the oxalic acid solution?
H2C2O4(aq) + 2 NaOH(aq) ➝ Na2C2O4(aq) + 2 H2O(l)
(a) 0.06631 M (b) 0.2337 M (c) 0.4675 M (d) 0.9350 M (e) none of these
Answer: (b)
2-84. What mass of sodium oxalate (Na2C2O4) is formed in this titration? (AW: C = 12.0, O = 16.0, Na = 23.0 amu)
(a) 0.0888 g (b) 0.313 g (c) 0.626 g (d) 1.25 g (e) none of the above
Answer: (b)
2-85. Calculate the volume of 0.0985 M sulfuric acid that would be needed to neutralize 10.89 mL of an 0.1043 M aqueous ammonia solution.
H2SO4(aq) + 2 NH3(aq) ➝ (NH4)2SO4(aq)
Answer: 5.77 mL
2-86. α-D-glucopyranose reacts with the periodate ion as follows,
C6H12O6(aq) + 5 IO4-(aq) ➝ 5 IO3-(aq) + 5 HCO2H(aq) + H2CO(aq)
Calculate the molarity of the glucopyranose solution if 25.0 mL of 0.750 M IO4- is required to consume 10.0 mL of this solution.
Answer: 0.375 M
2-87. Oxalic acid reacts with the chromate ion in acidic solution as follows,
3 H2C2O4(aq) + 2 CrO42-(aq) + 10 H+(aq) ➝ 6 CO(g) + 2 Cr3+(aq) + 8 H2O(l)
If 10.0 mL of oxalic acid consumes 40.0 mL of 0.0250 M CrO42-solution, what is the molarity of the oxalic acid solution?
Answer: 0.15 M
2-88. Assume that 6.70 mL of a Ca(OH)2 solution are added to 17.50 mL of 0.3750 M H2SO4. After this is done, the solution is still acidic and the excess H2SO4 s titrated with 0.2750 M KOH. If 15.00 mL of the KOH solution are needed to reach neutrality, what is the concentration of the Ca(OH)2 solution?
H2SO4(aq) + Ca(OH)2(aq) ➝ Ca2+(aq) + SO42-(aq) + 2 H2O(l)
H2SO4(aq) + 2 KOH(aq) ➝ 2 K+(aq) + SO42-(aq) + 2 H2O(l)
(a) 0.0336 M (b) 0.363 M (c) 0.670 M (d) 0.725 M (e) 1.34 M
Answer: (b)
2-89. How much HgS precipitate forms when 113 mL of a 0.75 M CaS solution is mixed with 52 mL of a 1.21 M Hg(NO3)2 solution? (AW: S = 32.06, Ca = 40.08, Hg = 200.6 amu)
(a) 6.30 g (b) 8.48 g (c) 14.6 g (d) 19.7 g (e) none of the above
Answer: (c)
2-90. If adding 22.7 mL of 0.6 M HCl to 1.00 L of a solution of AgNO3 precipitated 1.95 g of AgCl, what was the concentration of the original AgNO3 solution? (A.W.: H = 1.008, Cl = 35.45, Ag = 107.87 amu)
AgNO3(aq) + HCl(aq) ➝ AgCl(s) + HNO3(aq)
(a) 1.36 x 10-5 M (b) 1.36 x 10-2 M (c) 0.027 M (d) 0.68 M (e) 13.6 M
Answer: (b)
2-91. A bronze gong brought back to W. Lafayette from the Philippines by an archaeologist who happens to be the wife of one of the professors in charge of this course was analyzed for copper. Over her dead body, a 2.50 g sample of the bronze was cut from the gong, and dissolved in sulfuric acid. The copper sulfate produced was mixed with KI to form CuI and the triiodide ion, I3-. The I3- ion was then titrated with thiosulfate, S2O32-.
Cu(s) + 2 H2SO4(aq) ➝ CuSO4(aq) + SO2(g)+ 2 H2O(l)
2 CuSO4(aq) + 5 I-(aq) ➝ 2 CuI(s) + I3-(aq) + 2 SO42-(aq)
I3-(aq) + 2 S2O32-(aq) ➝ 3 I-(aq) + S4O62-(aq)
If it took 31.5 mL of 1.00 M thiosulfate for this titration, how many moles of copper were present in the original 2.5 g sample?
(a) 0.0105 (b) 0.0158 (c) 0.0315 (d) 0.0630 (e) 0.0945
Answer: (c)
2-92. What was the percent by weight of copper in this gong? (AW: O = 16.0, S = 32.1, K = 39.1, Cu = 63.5, I = 126.9 amu)
(a) less than 50% (b) between 50 and 60% (c) between 60 and 75%
(d) between 75 and 85% (e) more than 85%
Answer: (d)
Use the following information to answer questions 93-95:
Solutions of the thiosulfate ion (S2O32-) can be standardized by titration with an iodate (IO3-) solution of known concentration. The S2O32- solution can then used to titrate the I3- ion produced when an unknown Cu2+ ion solution is treated with excess I- ion, and thereby determine the concentration of the Cu2+ ion in this solution. Equations for the various reactions are shown below.
Standardization of S2O32-
A: IO3-(aq) + 8 I-(aq) + 6 H+(aq) ➝ 3 I3-(aq) + 3 H2O(l)
B: I3-(aq) + 2 e- ➝ 3 I-(aq)
C: 2 S2O32-(aq) ➝ S4O62-(aq) + 2 e-
D: overall: IO3-(aq) + 6 S2O32-(aq) + 6 H+(aq) ➝ I-(aq) + 3 S4O62-(aq) + 3 H2O(l)
Reaction of Cu2+ with I-
E: 2 Cu2+(aq) + 3 I-(aq) ➝ 2 Cu+(aq) + I3-(aq)
F: Cu+(aq) + I-(aq) ➝ CuI(s)
G: overall: 2 Cu2+(aq) + 5 I-(aq) ➝ 2 CuI(s) + I3-(aq)
Reaction of I3- with S2O32-
H: I3 -(aq) + 2 S2O32-(aq) ➝ 3 I-(aq) + S4O62-(aq)
2-93. Which of these reactions doesn’t involve oxidation-reduction?
(a) A (b) B (c) E (d) F (e) G
Answer: (d)
2-94. If 22.25 mL of 0.125 M KIO3 s required to react with 50.00 mL of the Na2S2O3 solution prepared in this experiment, what is the molarity of the S2O32- ion in the sodium thiosulfate solution?
(a) 2.78 x 10-3 M (b) 1.67 x 10-2 M (c) 5.56 x 10-2 M (d) 3.34 x 10-1 M
(e) none of these is correct
Answer: (d)
2-95. How many grams of Cu2+ are present in the unknown sample if 0.054 moles of S2O32- are required to react with the I3- ion produced by the unknown copper solution?
(a) 0.027 g (b) 0.054 g (c) 3.4 g (d) 6.8 g (e) 14 g
Answer: (c)
2-96. On April 19, 1995, a bomb exploded, killing 168 people, wounding hundreds of others and destroying the Alfred P. Murrah Federal Building in Oklahoma City. On August 10, 1998, a pick-up truck loaded with a similar explosive was driven up the steps of the Tippencanoe County Courthouse in Lafayette. The explosive mixture used in both incidents contains ammonium nitrate, NH4NO3, which is commonly used as a fertilizer. What is the molarity of concentrated nitric acid, HNO3(aq), if it takes 5.63 mL of the acid to consume 15.00 mL of 6.00 M NH3(aq)?
NH3(aq) + HNO3(aq) ⇌ NH4NO3(aq)
(a) Less than 1 M (b) Between 1 and 5 M (c) Between 5 and 10 M
(d) Between 10 and 15 M (e) More than 15 M
Answer: (e)
2-97. Concentrated sulfuric acid (H2SO4) has a density of 1.84 g/cm3 and this solution is 96% sulfuric acid by weight. (The rest is water.) What is the molarity of this solution?
(a) Less than 1 M (b) Between 1 and 5 M (c) Between 5 and 10 M
(d) Between 10 and 15 M (e) More than 15 M
Answer: (e)
2-98. One way of determining blood alcohol levels is by titrating a sample of blood according to the following net ionic equation.
C2H5OH + 2 Cr2O72- + 16 H+ ➝ 2 CO2 + 4 Cr3+ + 11 H2O
If 8.76 mL of 0.04988 M Cr2O72- is required for the titration of a 10.00-mL sample of blood, what was the molarity of the alcohol present in the blood sample?
(a) less than 0.01 M (b) between 0.01 and 0.03 M (c) between 0.03 and 0.06 M (d) between 0.06 and 0.09 M (e) more than 0.1 M
Answer: (b)
2-99. Long before we had million-dollar instruments to study the structure of carbohydrates this information had to be collected by more arduous and much more time-consuming methods, such as a seemingly endless array of titrations. One of the titrations that was used for this purpose involved the reaction between carbohydrates and the periodate ion (IO4-). For years, alcoholics have believed that one way of overcoming a hangover was to drink a Bloody Mary because tomato juice is a good source of the sugar known as fructose, which supposedly helps people recover from alcoholic binges. The reaction between fructose and the periodate ion is described by the following equation.
C6H12O6(aq) + 5 IO4-(aq) ➝ 5 IO3-(aq) + 5 HCO2H(aq) + H2CO(aq)
Calculate the molarity of a fructose solution if 22.35 mL of 1.050 M IO4- is required to consume 12.56 mL of this solution.
(a) less than 0.25 M (b) between 0.25 M and 0.5 M (c) between 0.5 M and 1.5 M
(d) between 1.5 M and 2 M (e) more than 2 M
Answer: (b)