CHAPTER 7:
MAKING AND BREAKING OF BONDS
The following questions often assume that a truncated table of standard-state thermodynamic data is attached to the exam.
Thermicity
7-1. Which of the following reactions is the most likely to give off energy?
(a) Al(s) ➝ Al(g)
(b) Al(g) ➝ Al3+(g) + 3 e-
(c) Al-(g) ➝ Al(g) + e-
(d) 2 Al(s) + conc HNO3(aq) ➝ 2 Al3+(aq) + 3 H2(g)
(e) all these reactions should give off energy.
Answer: (d)
7-2. Which of the following reactions is most likely to give off energy?
(a) Mg(s) ➝ Mg(g)
(b) Mg(g) ➝ Mg2+(g) + 2 e-
(c) MgCl(s) ➝ Mg2+(g) + 2 Cl-(g)
(d) Mg(s) + 2 HCl(aq) ➝ Mg2+(aq) + 2 Cl-(aq) + H2(g)
(e) all these reactions give off energy
Answer: (d)
7-3. Which of the following reactions is the most likely to be exothermic?
(a) H2(g) ➝ 2 H(g)
(b) 2 F(g) ➝ F2(g)
(c) C(s) ➝ C(g)
(d) CCl4(g) ➝ C(g) + 4 Cl(g)
(e) HCl(g) ➝ H(g) + Cl(g)
Answer: (b)
7-4. Which of the following reactions is the most likely to be exothermic?
(a) CaCO3(s) ➝ CaO(s) + CO2(g)
(b) Ca(g) ➝ Ca2+(g) + 2 e-
(c) Cl2(g) ➝ 2 Cl(g)
(d) Cl(g) + e- ➝ Cl-(g)
(e) CaCl2(g) ➝ Ca2+(g) + 2 Cl-(g)
Answer: (d)
7-5. Which of the following reactions is the most likely to be endothermic?
(a) 2 Na(s) + 2 H2O(l) ➝ 2 Na+(aq) + 2 OH-(aq) + H2(g)
(b) 2 Mg(s) + O2(g) ➝ 2 MgO(s)
(c) 2 NaCl(s) ➝ 2 Na(s) + Cl2(g)
(d) Na+(g) + e- ➝ Na(g)
Answer: (c)
7-6. Which of the following reactions is the most likely to be endothermic?
(a) Mg(s) ➝ Mg(g)
(b) Na+(g) + Cl-(g) ➝ NaCl(s)
(c) Na+(g) + e- ➝ Na(g)
(d) H+(aq) + OH-(aq) ➝ H2O(l)
(e) H2(g) + 1/2 O2(g) ➝ H2O(l)
Answer: (a)
7-7. Use your understanding of the bonding in the reactants and products to explain why the following reaction is exothermic.
2 Mg(s) + O2(g) ➝ 2 MgO(s)
Answer: The ionic bond in MgO must be stronger than the
mixture of metallic and covalent bonds in Mg metal and O2 gas.
Enthalpy
7-8. At what temperature are standard-state enthalpy of reaction measurements most often made?
(a) 0 K (b) 273.15 K (c) 0°C (d) 25°C (e) more than one of the above
Answer: (d)
7-9. For which of the following substances is the enthalpy of formation, ΔHf°, equal to zero?
(a) H2(l) (b) H2O(g) (c) O3(g) (d) F2(g) (e) Na(g)
Answer: (d)
7-10. For which of the following substances is the enthalpy of formation, ΔHf°, equal to zero?
(a) H2O(l) (b) H2O(s) (c) Cl(g) (d) P4(s) (e) CO2(g)
Answer: (d)
7-11. How much energy is required to heat 10.0 grams of gold from room temperature (20.0°C) to the temperature of boiling water (100.0°C) if the specific heat of gold is 0.129 J/g°C?
(a) 1.29 J (b) 10.3 J (c) 103 J (d) 6,200 J (e) none of the above
Answer: (c)
7-12. A piece of copper metal weighing 145 grams was heated to 100°C and then dropped into 250 grams of water at 25°C. The copper metal cooled down and the water became warmer until both were at a temperature of 28.8°C. Calculate the amount of heat absorbed by the water. Assuming that the heat lost by the copper was absorbed by the water, what is the molar heat capacity of copper metal? (CH2O = 75.376 J/mol-K)
(a) between 0 and 10 J/mol-K (b) between 10 and 20 J/mol-K
(c) between 20 and 30 J/mol-K (d) between 30 and 40 J/mol-K
(e) more than 40 J/mol-K
Answer: (c)
7-13. For which reaction is ΔH roughly equal to ΔE?
(a) 2 H2(g) + O2(g) ➝ 2 H2O(g)
(b) Pb(NO3)2(s) + 2 Kl(s) ➝ PbI2(s) + 2 KNO3(s)
(c) 2 Na(s) + 2 H2O(l) ➝ 2 Na+(aq) + 2 OH-(aq) + H2(g)
(d) NaOH(s) + CO2(g) ➝ NaHCO3(s)
(e) none of the above
Answer: (b)
7-14. For which of the following reactions is the change in the enthalpy of the system more or less equal to the change in the internal energy?
(a) H2 reacting with O2 to form H2O in a bomb calorimeter
(b) solid Pb(NO3)2 reacting with solid KI to form solid Pbl2 and solid KNO3
(c) an aqueous solution of HCl reacting with an aqueous solution of NaH to form an aqueous solution of NaCl and H2 gas.
(d) CO2 gas reacting with solid NaOH to form solid NaHCO3.
Answer: (b)
7-15. How much heat is produced by mixing 50.0 mL of 1.0 M HBr at 25.6°C with 50.0 mL of 1.0 M KOH at 25.6°C if this reaction produces 100 mL of a solution with a temperature of 32.3°C? (Assume the heat capacity of water is 4.18 J/g-°C and the density of these solutions is 1.00 g/cm3)
(a) 6.7 J (b) 670 J (c) 1400 J (d) 2800 J (e) 5600 J
Answer: (d)
7-16. Using the data in the previous question, calculate ΔH for the reaction
HBr(aq) + KOH(aq) ➝ KBr(aq) + H2O(l)
(a) -1.4 kJ (b) -2.8 kJ (c) -28 kJ (d) -56 kJ (e) 112 kJ
Answer: (d)
7-17. If mixing 50.0 mL of 1.0 M HBr and 50.0 mL of 1.0 M KOH produces a temperature increase of 6.70°C, then mixing 100 mL of 1.0 M HBr and 100 mL of 1.0 M KOH will produce a temperature increase of:
(a) 1.68°C (b) 3.35°C (c) 6.70°C (d) 13.4°C (e) impossible to predict
Answer: (c)
7-18. How much heat is produced when 0.200 mole of H2(g) reacts with 0.300 mole of Cl2(g) if the enthalpy of reaction for the production of one mole of HCl(g) is -92.3 kJ/mol?
(a) 18.5 kJ (b) 27.7 kJ (c) 36.9 kJ (d) 55.4 kJ (e) 92.3 kJ
Answer: (c)
7-19. How much energy is given off when 4.80 g of carbon are burned to produce carbon dioxide, if ΔHrxn° for the combustion of carbon to form CO2 is -394 kJ/mol?
(a) 82.1 kJ (b) 158 kJ (c) 394 kJ (d) 985 kJ (e) 1.89 x 103 kJ
Answer: (b)
7-20. Calculate ΔHrxn° in kJ/mol for the following reaction if 1.00 gram of magnesium reacts with excess fluorine to give off 46.22 kJ of heat.
Mg(s) + F2(g) ➝ MgF2(s)
(a) less than 300 kJ/mol (b) between 300 and 600 kJ/mol
(c) between 600 and 900 kJ/mol (d) between 900 and 1200 kJ/mol
(e) more than 1200 kJ/mol
Answer: (d)
Hess's Law
7-21. Use the following data
2 H2(g) + O2(g) ➝ 2 H2O(g)ΔH° = -483.6 kJ/molrxn
2 H2(g) + O2(g) ➝ 2 H2O(l) ΔH° = -571.6 kJ/molrxn
to calculate ΔH° for the reaction
H2O(l) ➝ H2O(g)
(a) -527.6 kJ (b) -44.0 kJ (c) 44.0 kJ d) 527.6 kJ (e) none of the above
Answer: (c)
7-22. What is ΔH° for the reaction: 2 CO(g) + O2(g) ➝ 2 CO2(g) if:
C(s) + 1/2 O2(g) ➝ CO(g)ΔH° = -111 kJ/molrxn
C(s) + O2(g) ➝ CO2(g) ΔH° = -393 kJ/molrxn
(a) -564 kJ (b) -282 kJ (c) 171 kJ (d) 282 kJ (e) 564 kJ
Answer: (a)
7-23. Calculate ΔH° for the reaction: C(s) + 2 H2(g) ➝ CH4(g) from the following data.
C(s) + O2(g) ➝ CO2(g) ΔH° = -393.9 kJ/molrxn
H2(g) + 1/2 O2(g) ➝ H2O(l)ΔH° = -285.8 kJ/molrxn
CH4(g) + 2 O2(g) ➝ CO2(g) + 2 H2O(l) ΔH° = -890.4 kJ/molrxn
(a) - 1855.7 kJ (b) -214.6 kJ (c) -75.1 kJ (d) 210.9 kJ (e) 1569.9 kJ
Answer: (c)
7-24. Given the following data
3 H2(g) + N2(g) ➝ 2 NH3(g) ΔH° = -92.4 kJ/molrxn
2 H2(g) + O2(g) ➝ 2 H2O(l) ΔH° = -571.7 kJ/molrxn
calculate ΔHrxn° for the following reaction:
4 NH3(g) + 3 O2(g) ➝ 2 N2(g) + 6 H2O(l)
(a) -1899.9 kJ (b) -1715.1 kJ (c) -1530.3 kJ (d) -479.3 kJ (e) 1530.3 kJ
Answer: (c)
7-25. Hydrogen peroxide is a good oxidizing agent because it is a good source of molecular oxygen: 2 H2O2(aq) ➝ 2 H2O(l) + O2(g). Calculate ΔHrxn° for this reaction from the enthalpies of the following reactions.
2 H2(g) + O2(g) ➝ 2 H2O(l) ΔH° = -571.66 kJ/molrxn
H2(g) + O2(g) ➝ H2O2(aq) ΔH° = -187.8 kJ/molrxn
(a) -759.5 kJ/mol (b) -383.9 kJ/mol (c) -196.0 kJ.mol
(d) -98.0 kJ/mol (e) 196.0 kJ/mol
Answer: (c)
7-26. Calculate ΔHrxn° for the reaction:
N2(g) + O2(g) ➝ 2 NO(g)
from the enthalpies of the following reactions.
N2(g) + 2 O2(g) ➝ 2 NO2(g)ΔH° = 66.4 kJ/molrxn
2 NO(g) + O2(g) ➝ 2 NO2(g) ΔH° = -114.2 kJ/molrxn
(a) -180.6 kJ/mol (b) -47.8 kJ/mol (c) 47.8 kJ/mol (d) 180.6 kJ/mol
(e) ΔH° is impossible to calculate from the information given
Answer: (d)
7-27. Calculate the heat of combustion of propane, C3H8,
C3H8(g) + 5 O2(g) ➝ 3 CO2(g) + 4 H2O(g)
from the enthalpies of the following reactions.
3 C(s) + 4 H2(g) ➝ C3H8(g)ΔH° = -103.85 kJ/molrxn
C(s) + O2(g) ➝ CO2(g)ΔH° = -393.51 kJ/molrxn
H2(g) + ½ O2(g) ➝ H2O(g)ΔH° = -241.83 kJ/molrxn
Answer: -2044.0 kJ/molrxn
7-28. Calculate ΔH° for the reaction: 2 N2(g) + 5 O2(g) ➝ 2 N2O5(g)
from the following enthalpy of reaction data.
N2(g) + 3 O2(g) + H2(g) ➝ 2 HNO3(aq) ΔH° = -207.4 kJ/molrxn
N2O5(g) + H2O(g) ➝ 2 HNO3(aq)ΔH° = 218.4 kJ/molrxn
2 H2(g) + O2(g) ➝ 2 H2O(g)ΔH° = -571.6 kJ/molrxn
Answer: -280.0 kJ/molrxn
7-29. What is the sign of the enthalpy of reaction for the reaction:
P4O6(s) + 2 O2(g) + 6 H2O(g) ➝ 4 H3PO4(s)
assuming that all compounds are present in their most stable state at 25°C and 1 atm pressure?
Compound ΔHac° (kJ/mol)
P4O6(s) -4393.7
O2(g) -498.34
H2O(g) -926.29
H3PO4(s) -3243.3
(a) positive (b) negative (c) impossible to determine from the data
Answer: (b)
7-30. What is the magnitude of the enthalpy of reaction in the previous question?
(a) less than 1000 kJ/mol (b) between 1000 and 1250 kJ/mol
(c) between 1250 and 1500 kJ/mol (d) between 1500 and 1750 kJ/mol
(e) more than 1750 kJ/mol
Answer: (e)
7-31. Calculate the energy required to transform 18 g of ice at 0°C to water vapor at 100°C using some or all of the data given below.
H2O(s) ➝ H2O(l) ΔH° = 6.03 kJ/molrxn
H2O(l) ➝ H2O(g)ΔH° = 40.67 kJ/molrxn
2 H2(g) + O2(g) ➝ 2 H2O(g)ΔH° = -484 kJ/molrxn
H2O(liquid, 0°C) ➝ H2O(liquid, 100°C)ΔH° = 7.53 kJ/molrxn
(a) 40.67 kJ (b) 46.70 kJ (c) 54.23 kJ (d) 296 kJ (e) none of these
Answer: (c)
Use the following standard enthalpies of atom combination to answer questions 32 through 36.
ΔHac° H2(g) = -435.30 kJ/mol ΔHac F2(g) = -157.98 kJ/mol
ΔHac° N2(g) = -945.41 kJ/mol ΔHac° C(s) = -716.68 kJ/mol
ΔHac° O2(g) = -498.34 kJ/mol ΔHac° NH3(g) = -1171.8 kJ/mol
ΔHac° H2O(g) = -926.3 kJ/mol ΔHac° HF(g) = -567.7 kJ/mol
7-32. What is ΔH° for the following reaction? 3 N(g) ➝ 3/2 N2(g)
(a) +710 kJ (b) -710 kJ (c) +1418 kJ (d) -1418 kJ (e) -473 kJ
Answer: (d)
7-33. What is the bond energy of N≡N?
(a) 237 kJ/mol (b) 158 kJ/mol (c) 473 kJ/mol (d) 946 kJ/mol (e) 1892 kJ/mol
Answer: (d)
7-34. What is the ΔH° for the following reaction as written?
4 NH3(g) + 3 O2(g) ➝ 2 N2(g) + 6 H2O(g)
(a) -1266 kJ (b) -1184 kJ (c) -196 kJ (d) +767 kJ (e) none of the above
Answer: (a)
7-35. What is the bond energy, H2N—H, for one of the N-H bonds in NH3(g)?
(a) 391 kJ/mol (b) 645 kJ/mol (c) 737 kJ/mol (d) 846 kJ/mol (e) 1173 kJ/moI
Answer: (a)
7-36. How much heat is absorbed or evolved when just enough H2(g) reacts with just enough F2(g) to produce 0.200 mol of HF(g)?
(a) 54 kJ of heat is absorbed (b) 54 kJ of heat is evolved
(c) 108 kJ of heat is absorbed (d) 108 kJ of heat is evolved
(e) 216 kJ of heat is absorbed
Answer: (b)
7-37. Calculate ΔH° for the following reaction from the data given below.
4 NH3(g) + 5 O2(g) ➝ 4 NO(g) + 6 H2O(g)
Compound ΔHac° (kJ/mol)
NH3(g) -1171.76
O2(g) -498.34
NO(g) -631.62
H2O(g) -926.29
(a) -105.5 kJ (b) -905.5 kJ (c) -1274.2 kJ
(d) -1996.2 kJ (e) none of the above
Answer: (b)
7-38. Calculate ΔH° for the reaction:
3 NO2(g) + H2O(l) ➝ 2 HNO3(aq) + NO(g)
from the following enthalpy of atom combination data.
Compound ΔHac° (kJ/mol)
NO2(g) -937.86
H2O(l) -970.30
HNO3(aq) -1645.22
NO(g) -631.62
(a) less than -1000 kJ/molrxn (b) between -1000 and -750 kJ/molrxn
(c) between -750 and -500 kJ/molrxn (d) between -500 and -250 kJ/molrxn
(e) between -250 and 0 kJ/molrxn
Answer: (e)
7-39. Both ethanol (CH3CH2OH) and methanol (CH3OH) have been considered as fuels for automobiles. Which is the better fuel, on a per gram basis, when burned with oxygen? (Hint: Draw Lewis structures for all molecules and show what bonds are broken and made.)
CompoundΔHac° (kJ/mol)
CH3CH2OH(g) -3223.53
CH3OH(g) -2037.11
O2(g) -498.34
H2O(g) -926.29
CO2(g) -1608.53
H2O(l) -970.30
Answer: 21 kJ/g CH3OH versus 28 kJ/g CH3CH2OH
7-40. The following reaction occurs when sucrose (cane sugar) is metabolized by the body.
C12H22O11(s) + 12 O2(g) ➝ 12 CO2(g) + 11 H2O(l)
Assume that ΔH° for this reaction is -5645 kJ/molrxn. What is the value of ΔHac° for sucrose?
CompoundΔHac° (kJ/mol)
O2(g) -498.34
CO2(g) -1608.53
H2O(l) -970.30
Answer: -18,350 kJ/mol
7-41. For which of the following reactions is the most heat evolved?
CH4(g) + 2 O2(g) ➝ CO2(g) + 2 H2O(g)
(methane)
2 CH3CH2CH2CH3(g) + 13 O2(g) ➝ 8 CO2(g) + 10 H2O(g)
(butane)
2 (CH3)2CHCH3(g) + 13 O2(g) ➝ 8 CO2(g) + 10 H2O(g)
(isobutane)
Answer: methane = 802 kJ/molrxn, butane = 2656 kJ/molrxn, isobutane = 2649 kJ/molrxn
7-42. Use enthalpies of atom combination to calculate ΔH° for the following reaction.
C(s) + H2O(g) ➝ CO(g) + H2(g)
What would happen to the magnitude of ΔH° if the reaction consumed liquid water instead of gaseous water?
Compound ΔHac° (kJ/mol)
C(s) -717
H2O(g) -926
H2O(l) -970
CO(g) -1076
H2(g) -435
Answer: 132 kJ/molrxn; it would become more positive
7-43. Write a balanced equation for the combustion of carbon disulfide to form carbon dioxide and sulfur dioxide, draw the Lewis structures of all reactants and products, and calculate ΔH° for this reaction.
_____ CS2(g) + _____ O2(g) ➝ _____ CO2(g) + _____ SO2(g)
Answer: -1105 kJ/mol
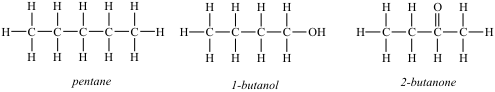
7-44. Consider the following data for heats of atom combination of three substances in the liquid and gaseous states.
Compound ΔHac° (liquid) ΔHac° (gas) ΔH(liq) - ΔH(gas)
(kJ/mol) (kJ/mol) (kJ/mol)
pentane -6373 -6347 26
1-butanol -6060 -6007 53
2-butanone -5793 -5761 32
The structures of these molecules are shown below.

Explain the relative magnitudes of the differences between the enthalpies of atomization of the liquid and gaseous states of these three compounds.
Answer: Intermolecular forces are largest in 1-butanol and smallest in pentane.