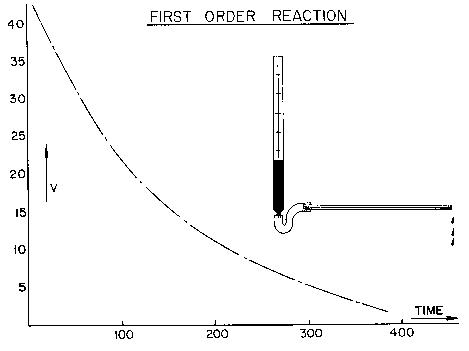
Chemical Concepts Demonstrated: Order of a reaction, first-order kinetics
Demonstration:
Capillary tubing is attached a buret filled with water and clamped in a horizontal
position.
Use a stop watch and record time vs. volume measurements as the water flows out of the
buret.
Observations:

Plotting volume versus time yields a slightly curved line.
Plotting the log of the volume versus the time yields a straight line.
Explanation:
Because the log of the volume versus the time yields a straight line when plotted, the capillary flow appears to be first-order.