- The first dish contains an iron nail.
- The second dish contains an iron nail with one end wrapped in copper wire connected to a piece of zinc metal.
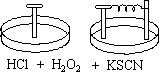
Chemical Concepts Demonstrated: Corrosion, cathodic protection, sacrificial anode
Demonstration:
Both dishes contain a solution of HCl, 3% H2O2, and KSCN.
|
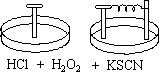 |
Observations:
The solution in the dish containing the lone nail turns a pink color,
and the intensity of the color increases over time. In the dish containing the
nail/zinc metal connection, a gas is produced on the surface of the metal, but the pink
color does not appear. When the connection between the nail and the zinc metal is
severed, the solution begins to turn pink.
Explanations (including important chemical equations):
The pink coloration is due to the formation of Fe(SCN)2+/Fe(SCN)2+ complexes. This indicates that the iron metal is being oxidized to the Fe3+ ion. This won't occur in the second dish as long as the nail is connected to the zinc metal strip. When the two metals are connected, the zinc undergoes oxidation to Zn 2+ at the same time that H + ions are reduced to H2 on the surface of the iron nail.