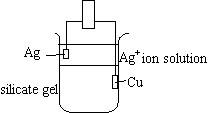
- A copper metal electrode is inside the gel.
- A metal ion solution (Ag+, Zn2+, or Pb2+) is poured over the gel.
- A corresponding metal electrode is placed in the solution.
Chemical Concepts Demonstrated: Galvanic and voltaic cells
Demonstration:
The silicate gel is made up of sodium silicate dissolved in water and a Cu2+ solution
made of Cu(NO3)2 * H2O dissolved in water with acetic
acid.
|
 |
Observations:
The gel allows the copper metal to act as a voltaic cell with the metal ion solution.
Explanation (including chemical equations):
The Ag +/Ag half-cell coupled with the Cu 2+ /Cu half-cell yields a standard-state cell potential of 0.46 V.
| anode: | Cu (s) ---> Cu 2+ (aq) + 2 e- | Eo = - 0.34 V |
| cathode: | Ag + (aq) + e- ---> Ag(s) | Eo = 0.80 V |
| Cu (s) + 2 Ag + (aq) ---> Cu 2+ (aq) + 2 Ag(s) | Eo cell = 0.46 V |
If the Ag +/Ag half-cell is replaced using the Pb 2+/Pb or the Zn 2+/ Zn half reactions, the leads to the voltmeter would need to be reversed in order to set up a volaic cell:
| anode: | Cu (s) ---> Cu 2+ (aq) + 2 e- | Eo = - 0.34 V |
| cathode: | Pb 2+ (aq) + 2 e- ---> Pb (s) | Eo = - 0.13 V |
| Cu (s) + Pb 2+ (aq) ---> Cu 2+ (aq) + Pb (s) | Eo cell = -0.47 V |
or:
| anode: | Cu (s) ---> Cu 2+ (aq) + 2 e- | Eo = - 0.34 V |
| cathode: | Zn 2+ (aq) + 2 e- ---> Zn (s) | Eo = - 0.76 V |
| Cu (s) + Zn 2+ (aq) ---> Cu 2+ (aq) + Zn (s) | Eo cell = -1.10 V |