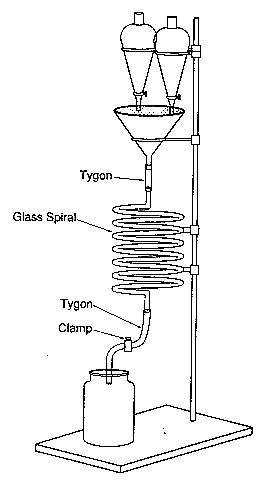
Chemical Concept Demonstrated: Chemiluminescence
Demonstration:
Solution A
|
Solution B
|
Pour solutions A and B simultaneously into the funnel. 
Observations:
The entire spiral gives off a singular glow.
Explanation:
Luminol is converted to a dianion in basic solution. This dianion is then oxidized with H2O2/Cu2+ to form N2 and an aminophthalate ion in an electronically excited state. In order to achieve an electronically unexcited state, the ion emits a photon of wavelength 424 nm.