Empirical Formula 1
(Calculation of the empirical formula from mass
data.)
Empirical Formula: The simplest ratio of the atoms
present in a molecule.
Problem:
Find the empirical formula for the oxide that contains 42.05 g of nitrogen and 95.95 g of oxygen.
Strategy:
As with most stoichiometry problems, it is necessary to work
in moles. The ratio of the moles of each element will provide the
ratio of the atoms of each element.
- Convert the mass of each element to moles of each element
using the atomic masses.
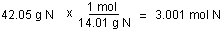
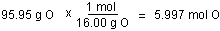
- Find the ratio or the moles of each element by dividing
the number of moles of each by the smallest number of
moles.
There are fewest moles of nitrogen, so assume one mole of
nitrogen in finding the ratio.
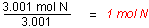
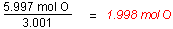
- Use the mole ratio to write the empirical
formula.
Since there cannot be partial atoms in the
empirical formula, the mole ratios must be whole numbers.
1.998 is sufficiently close to 2 that we can round. Thus
the empirical formula is:NO2


