The Chemistry of Hemoglobin and Myoglobin
At one time or another, everyone has experienced the momentary sensation of having to stop, to "catch one's breath," until enough O2 can be absorbed by the lungs and transported through the blood stream. Imagine what life would be like if we had to rely only on our lungs and the water in our blood to transport oxygen through our bodies.
O2 is only marginally soluble (< 0.0001 M)
in blood plasma at physiological pH. If we had to rely on the
oxygen that dissolved in blood as our source of oxygen, we would
get roughly 1% of the oxygen to which we are accustomed.
(Consider what life would be like if the amount of oxygen you
received was equivalent to only one breath every 5 min, instead
of one breath every 3 s.) The evolution of forms of life even as
complex as an earthworm required the development of a mechanism
to actively transport oxygen through the system. Our blood stream
contains about 150 g/L of the protein known as hemoglobin
(Hb), which is so effective as an oxygen-carrier that the
concentration of O2 in the blood stream reaches 0.01 M
![]() the same concentration as air. Once the
Hb-O2 complex reaches the tissue that consumes oxygen,
the O2 molecules are transferred to another protein
the same concentration as air. Once the
Hb-O2 complex reaches the tissue that consumes oxygen,
the O2 molecules are transferred to another protein ![]() myoglobin (Mb)
myoglobin (Mb) ![]() which transports oxygen through the muscle tissue.
which transports oxygen through the muscle tissue.
The site at which oxygen binds to both hemoglobin and myoglobin is the heme shown in the figure below.
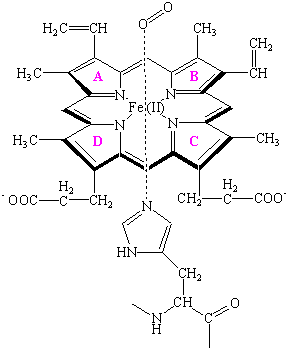
At the center of the heme is an Fe(II) atom. Four of the six coordination sites around this atom are occupied by nitrogen atoms from a planar porphyrin ring. The fifth coordination site is occupied by a nitrogen atom from a histidine side chain on one of the amino acids in the protein. The last coordination site is available to bind an O2 molecule. The heme is therefore the oxygen-carrying portion of the hemoglobin and myoglobin molecules. This raises the question: What is the function of the globular protein or "globin" portion of these molecules?
The structure of myoglobin suggests that the oxygen-carrying heme group is buried inside the protein portion of this molecule, which keeps pairs of hemes group from coming too close together. This is important, because these proteins need to bind O2 reversibly and the Fe(II) heme, by itself, cannot do this. When there is no globin to protect the heme, it reacts with oxygen to form an oxidized Fe(III) atom instead of an Fe(II)-O2 complex.
Hemoglobin consists of four protein chains, each about the
size of a myoglobin molecule, which fold to give a structure that
looks very similar to myoglobin. Thus, hemoglobin has four
separate heme groups that can bind a molecule of O2.
Even though the distance between the iron atoms of adjacent hemes
in hemoglobin is very large![]() between 250 and 370
nm
between 250 and 370
nm ![]() the act of binding an O2
molecule at one of the four hemes in hemoglobin leads to a
significant increase in the affinity for O2 binding at
the other hemes.
the act of binding an O2
molecule at one of the four hemes in hemoglobin leads to a
significant increase in the affinity for O2 binding at
the other hemes.
This cooperative interaction between different binding sites makes hemoglobin an unusually good oxygen-transport protein because it enables the molecule to pick up as much oxygen as possible once the partial pressure of this gas reaches a particular threshold level, and then give off as much oxygen as possible when the partial pressure of O2 drops significantly below this threshold level. The hemes are much too far apart to interact directly. But, changes that occur in the structure of the globin that surrounds a heme when it picks up an O2 molecule are mechanically transmitted to the other globins in this protein. These changes carry the signal that facilitates the gain or loss of an O2 molecule by the other hemes.
Drawings of the structures of proteins often convey the
impression of a fixed, rigid structure, in which the side-chains
of individual amino acid residues are locked into position.
Nothing could be further from the truth. The changes that occur
in the structure of hemoglobin when oxygen binds to the hemes are
so large that crystals of deoxygenated hemoglobin shatter when
exposed to oxygen. Further evidence for the flexibility of
proteins can be obtained by noting that there is no path in the
crystal structures of myoglobin and hemoglobin along which an O2
molecule can travel to reach the heme group. The fact that these
proteins reversibly bind oxygen suggests that they must undergo
simple changes in their conformation ![]() changes
that have been called breathing motions.
changes
that have been called breathing motions.![]() that open up and then close down the pathway along which an O2
molecule travels as it enters the protein. Computer
simulations of the motion within proteins suggests that the
interior of a protein has a significant "fluidity,"
with groups moving within the protein by as much as 20 nm.
that open up and then close down the pathway along which an O2
molecule travels as it enters the protein. Computer
simulations of the motion within proteins suggests that the
interior of a protein has a significant "fluidity,"
with groups moving within the protein by as much as 20 nm.