Practice Problem 7
Name the straight-chain constitutional and stereoisomers of pentene (C5H10).
Solution
There are two constitutional isomers of pentene in which all the carbon atoms lie in the same chain.
![]()
There are no cis/trans isomers of l-pentene because there is only one way of arranging the substituents around the double bond.
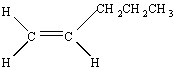
Cis and trans isomers are possible, however, for 2-pentene.
