
Practice Problem 8
Determine whether the following compound should be described as the Z or E isomer of 3-methyl-2-pentene.

Solution
There is no doubt about the priority of the CH3 and H substituents on the second carbon atom in this molecule.
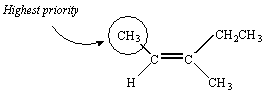
There is no way to assign priority to the atoms bound to the third carbon in this molecule -- they are both carbon atoms. We therefore proceed down the substituent. The next atoms we encounter in the CH3 group are all hydrogen atoms. But the next atoms in the CH2CH3 substituent are two hydrogen atoms and a carbon atom. Thus, the CH2CH3 group has the higher priority.
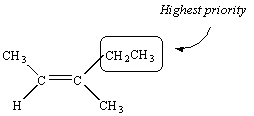
We therefore conclude that the two substituents with the highest priority are on the same side of the C=C double bond.

This compound is therefore the Z isomer and it would be named as follows: (Z)-3-methyl-2-pentene.