Definitions of Terms
| Linear, Branched, and Cross-linked Polymers | Homopolymers and Copolymers | Tacticity |
| Addition Versus Condensation Polymers | Elastomers | |
Linear, Branched, and Cross-linked Polymers
The term polymer is used to describe compounds with relatively large molecular weights formed by linking together many small monomers. Polyethylene, for example, is formed by polymerizing ethylene molecules.
![]()
Polyethylene is called a linear or straight-chain polymer because it consists of a long string of carbon-carbon bonds. These terms are misleading because the geometry around each carbon atom is tetrahedral and the chain is neither linear nor straight, as shown in the figure below.

As the polymer chain grows, it folds back on itself in a random fashion to form structures such as the one shown in the figure below.

Polymers with branches at irregular intervals along the polymer chain are called branched polymers (see figure below).

These branches make it difficult for the polymer molecules to pack in a regular array, and therefore make the polymer less crystalline. Cross-linked polymers contain branches that connect polymer chains, as shown in the figure below.
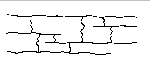
At first, adding cross-links between polymer chains makes the polymer more elastic. The vulcanization of rubber, for example, results from the introduction of short chains of sulfur atoms that link the polymer chains in natural rubber. As the number of cross-links increases, the polymer becomes more rigid.
The decision to classify a polymer as branched or cross-linked is based on the extent to which the side-chains on the polymer backbone link adjacent polymer chains. The easiest way to distinguish between these categories is to study the effect of various solvents on the polymer. Branched polymers are often soluble in one or more solvents because it is possible to separate the polymer chains. Cross-linked polymers are insoluble in all solvents because the polymer chains are tied together by strong covalent bonds.
Linear and branched polymers form a class of materials known as thermoplastics. These materials flow when heated and can be molded into a variety of shapes which they retain when they cool. Heavy cross-linking produces materials known as thermoset plastics. Once the cross-links form, these polymers take on a shape that cannot be changed without destroying the plastic. The polypropylene used in the plastic chairs that fill so many classrooms is a thermoplastic; as you lean back on the chair you can feel it give. The plastic case in which early radios were placed is an example of a thermoset plastic; it had a tendency to shatter rather than bend if the radio was dropped on the floor.
| Practice Problem 1: Polyethylene can be obtained in two different forms. High-density polyethylene (0.94 g/cm3) is a linear polymer. Low-density polyethylene (0.92 g/cm3) is a branched polymer with short side-chains on 3% of the atoms along the polymer chain. Explain how the structure of these polymers gives rise to the difference in their densities. |
Polyethylene is an example of a homopolymer that is formed by polymerizing a single monomer. Copolymers are formed by polymerizing more than one monomer. Ethylene (CH2=CH2) and propylene (CH2=CHCH3) can be copolymerized, for example, to produce a polymer that has two kinds of repeating units.

Copolymers are classified on the basis of the way monomers are arranged along the polymer chain, as shown in the figure below.
| Random copolymer |
| Regular copolymer |
| Block copolymer |
| Graft copolymer |
Random copolymers contain repeating units arranged in a purely random fashion. Regular copolymers contain a sequence of regularly alternating repeating units. The repeating units in block copolymers occur in blocks of different lengths. Graft copolymers have a chain of one repeating unit grafted onto the backbone of another.
Polymers with regular substituents on the polymer chain possess a property known as tacticity (from the Latin tacticus, fit for arranging). Tacticity results from the different ways in which the substituents can be arranged on the polymer backbone (see figure below).
 |
Atactic polypropylene |
 |
Syndiotactic polystyrene |
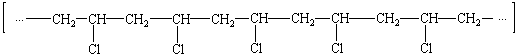 |
Isotactic poly(vinyl chloride) |
When the substituents are arranged in an irregular, random fashion, the polymer is atactic (literally, no arrangement). When the substituents are all on the same side of the chain, the polymer is isotactic (literally, the same arrangement). If the substituents alternate regularly from one side of the chain to the other, the polymer is syndiotactic.
| Practice Problem 2: Atactic polypropylene is a soft, rubbery material with no commercial value. The isotactic polymer is a rigid substance with an excellent resistance to mechanical stress. Explain the difference between the physical properties of these two forms of polypropylene. |
Addition Versus Condensation Polymers
Polyethylene, polypropylene, and poly(vinyl chloride) are addition polymers formed by adding monomers to a growing polymer chain. Addition polymers can be recognized by noting that the repeating unit always has the same formula as the monomer from which the polymer is formed.
| Polyethylene | ||
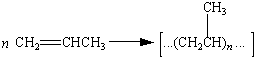 |
Polypropylene | |
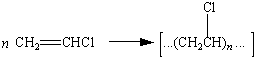 |
Poly(vinyl chloride) |
To condense means to make something more dense, or compact. Polymers formed when a small molecule condenses out during the polymerization reaction are therefore called condensation polymers. Silicone, for example, is a condensation polymer formed by polymerizing (CH3)2Si(OH)2. Each time a monomer is added to the polymer chain, a molecule of water is condensed out, as shown in the figure below. Note that the repeating unit in a condensation polymer is inevitably smaller than the monomer from which it is made.
| Practice Problem 3: Classify the products of the following reactions as either addition or condensation polymers. (a) Poly(methyl methacrylate), sold as Lucite or Plexiglass.
(b) Nylon 6
|
Elastomers are polymers that have the
characteristic properties of rubber ![]() they are both flexible and
elastic. To be elastic, a polymer must meet the following
criteria.
they are both flexible and
elastic. To be elastic, a polymer must meet the following
criteria.
 |
Stretch |  |
| Relax |
We can understand these requirements by taking a closer look at the chemistry of natural rubber, which is a polymer of a C5H8 hydrocarbon known as isoprene.
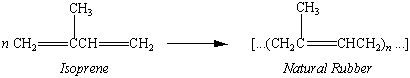
The double bonds in natural rubber are all in the cis form, which gives rise to long, flexible molecules. The force of attraction between polymer chains is relatively small, so the polymer can curl back into its original shape after the molecules have been oriented by stretching. By adding sulfur to natural rubber it is possible to introduce a small number of cross-links between these polymer chains that hold these chains together when the polymer is stretched.
At first glance, it might seem easy to make synthetic rubber. All we have to do is find a suitable catalyst that can polymerize isoprene. The task is made more difficult by the fact that the cis isomer of isoprene rearranges into the trans isomer during polymerization and the trans isomer of polyisoprene, which is known as gutta percha, is not elastic. It is therefore important to control the geometry around the C=C double bond during polymerization to make sure that as few of these bonds as possible are converted to the trans geometry. Until recently, this wasn't possible, and other approaches to making synthetic rubber were necessary.
The first solution to this problem involved polymerizing 2-chloro-1,3-butadiene, or "chloroprene," to form the first major synthetic rubber, neoprene.
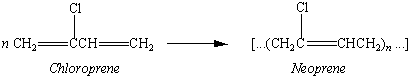
This approach is still used to produce a copolymer of 75% butadiene and 25% styrene known as styrene-butadiene rubber (SBR). Roughly 40% of the rubber used in the world today is SBR; another 35% is natural rubber that has been treated with sulfur.
The effect of cross-linking on elastomers can be demonstrated with a pair of rubber balls available from Flinn Scientific (catalog #AP1971). One of these balls is a polybutadiene rubber that contains an unusually large amount of sulfur. Because the polymer chains are extensively cross-linked, this ball dissipates very little energy in the form of heat when it bounces. It is therefore extremely resilient when bounced on the floor.
The other ball is a styrene-butadiene copolymer with much less cross-linking. When dropped on the floor, the ball seems to "die." This copolymer is used in applications where an energy-absorbing medium is desired, such as automobile tires which must absorb some of the energy associated with the bumps we encounter as drive down the highway.