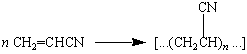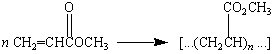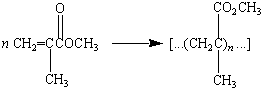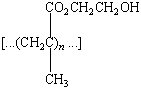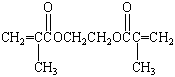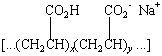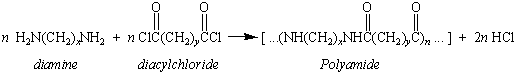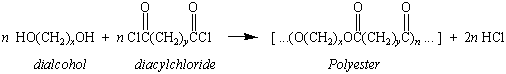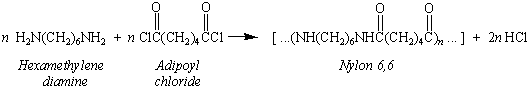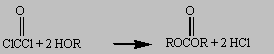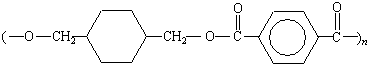Types of Polymers
Addition Polymers
Addition polymers such as polyethylene, polypropylene,
poly(vinyl chloride), and polystyrene are linear or branched
polymers with little or no cross-linking. As a result, they are
thermoplastic materials, which flow easily when heated and can be
molded into a variety of shapes. The structures, names, and trade
names of some common addition polymers are given in the table
below.
Common Addition Polymers
| Structure |
|
Chemical Name |
|
Trade Name or
Common Name |
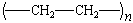 |
|
polyethylene |
|
|
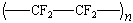 |
|
poly(tetrafluoroethylene) |
|
Teflon |
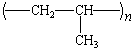 |
|
polypropylene |
|
Herculon |
 |
|
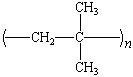
polyisobutylene |
|
butyl rubber |
 |
|
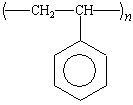
polystyrene |
|
|
 |
|
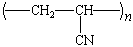
polyacrylonitrile |
|
Orlon |
 |
|
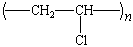
poly(vinyl chloride) |
|
PVC |
 |
|
poly(methyl acrylate) |
|
|
 |
|
poly(methyl methacrylate) |
|
Plexiglas, Lucite |
 |
|
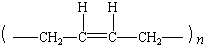
polybutadiene |
|
|
 |
|
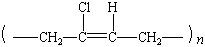
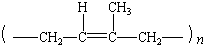
polychloroprene |
|
neoprene |
 |
|
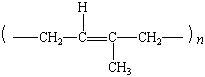
poly(cis-1,4-isoprene) |
|
natural rubber |
 |
|
poly(trans-1,4-isoprene) |
|
gutta percha |

Polyethylene
Low-density polyethylene (LDPE) is produced by free-radical
polymerization at high temperatures (200C) and high pressures
(above 1000 atm). The high-density polymer (HDPE) is obtained
using Ziegler-Natta catalysis at temperatures below 100C and
pressures less than 100 atm. More polyethylene is produced each
year than any other plastic. About 7800 million pounds of
low-density and 4400 million pounds of high-density polyethylene
were sold in 1980. Polyethylene has no taste or odor and is
lightweight, nontoxic, and relatively inexpensive. It is used as
a film for packaging food, clothing, and hardware. Most
commercial trash bags, sandwich bags, and plastic wrapping are
made from polyethylene films. Polyethylene is also used for
everything from seat covers to milk bottles, pails, pans, and
dishes.

Polypropylene
The isotactic polypropylene from Ziegler-Natta-catalyzed
polymerization is a rigid, thermally stable polymer with an
excellent resistance to stress, cracking, and chemical reaction.
Although it costs more per pound than polyethylene, it is much
stronger. Thus, bottles made from poly-propylene can be thinner,
contain less polymer, and cost less than conventional
polyethylene products. Polypropylene's most important impact on
today's college student takes the form of the plastic stackable
chairs that abound on college campuses.

Poly(tetrafluoroethylene)
Tetrafluoroethylene (CF2=CF2) is a gas
that boils at -76C and is therefore stored in cylinders at high
pressure. In 1938 Roy Plunkett received a cylinder of
tetrafluoroethylene that didn't deliver as much gas as it should
have. Instead of returning the cylinder, he cut it open with a
hacksaw and discovered a white, waxy powder that was the first
polytetrafluoroethylene polymer. After considerable effort, a
less fortuitous route to this polymer was discovered, and
polytetrafluoro-ethylene, or Teflon, became commercially
available.
Teflon is a remarkable substance. It has the best resistance
to chemical attack of any polymer, and it can be used at any
temperature between -73°C and 260°C with no effect on its
properties. It also has a very low coefficient of friction. (In
simpler crude terms, it has a waxy or slippery touch.) Even
materials as "sticky" as rubber, adhesives, bread
dough, and candy won't insects that stick to a Teflon-coated
surface. Teflon is so slippery that it has even been sprayed on
plants, so that might prey on the plants fall off.

Poly(vinyl
Chloride) and Poly(vinylidene Chloride)
Chlorine is one of the top ten industrial chemicals in the US  more than 20
billion pounds are produced annually. About 20% of this chlorine
is used to make vinyl chloride (CH2=CHCl) for the
production of poly(vinyl chloride), or PVC. The chlorine
substituents on the polymer chain make PVC more fire-resistant
than polyethylene or polypropylene. They also increase the force
of attraction between polymer chains, which increases the
hardness of the plastic. The properties of PVC can be varied over
a wide range by adding plasticizers, stabilizers, fillers, and
dyes, making PVC one of the most versatile plastics.
more than 20
billion pounds are produced annually. About 20% of this chlorine
is used to make vinyl chloride (CH2=CHCl) for the
production of poly(vinyl chloride), or PVC. The chlorine
substituents on the polymer chain make PVC more fire-resistant
than polyethylene or polypropylene. They also increase the force
of attraction between polymer chains, which increases the
hardness of the plastic. The properties of PVC can be varied over
a wide range by adding plasticizers, stabilizers, fillers, and
dyes, making PVC one of the most versatile plastics.
A copolymer of vinyl chloride (CH2=CHCl) and
vinylidene chloride (CH2=CCl2) is sold
under the trade name Saran. The same increase in the force of
attraction between polymer chains that makes PVC harder than
polyethylene gives thin films of Saran a tendency to
"cling."

Acrylics
Acrylic acid is the common name for 2-propenoic acid: CH2=CHCO2H.
Acrylic fibers such as Orlon are made by polymerizing a
derivative of acrylic acid known as acrylonitrile.
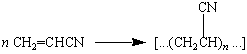 |
|
Polyacrylonitrile |
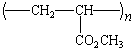
Other acrylic polymers are formed by polymerizing an ester of
this acid, such as methyl acrylate.
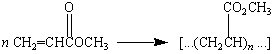 |
|
Poly(methyl acrylate) |
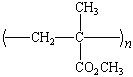
One of the most important acrylic polymers is poly(methyl
methacrylate), or PMMA, which is sold under the trade names
Lucite and Plexiglass.
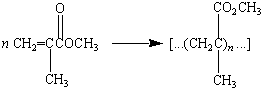 |
|
Poly(methyl methacrylate), PMMA |
PMMA is a lightweight, crystal-clear, glasslike polymer used
in airplane windows, taillight lenses, and light fixtures.
Because it is hard, stable to sunlight, and extremely durable,
PMMA is also used to make the reflectors embedded between lanes
of interstate highways.
The unusual transparency of PMMA makes this polymer ideal for
hard contact lenses. Unfortunately, PMMA is impermeable to oxygen
and water. Oxygen must therefore be transported to the cornea of
the eye in the tears and then passed under the contact lens each
time the eye blinks. Soft plastic lenses that pass both oxygen
and water are made by using ethylene glycol dimethacrylate to
crosslink poly(2-hydroxyethyl methacrylate).
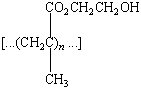 |
|
Poly(2-hydroxyethylmethacrylate) |
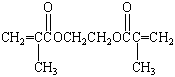 |
|
Ethylene glycol dimethacrylate |
An interesting polymer can be prepared by copolymerizing a
mixture of acrylic acid and the sodium salt of acrylic acid. The
product of this reaction has the following structure.
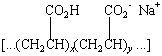 |
|
Sodium polyacrylate |
The difference between the Na+ ion concentration
inside the polymer network and in the solution in which the
polymer is immersed generates an osmotic pressure that draws
water into the polymer. The amount of liquid that can be absorbed
depends on the ionic strength of the solution  the total
concentration of positive and negative ions in the solution. This
polymer can absorb 800 times it own weight of distilled water,
but only 300 times its weight of tap water. Because the ionic
strength of urine is equivalent to an 0.1 M NaCl
solution, this superabsorbant polymer, which can be found in
disposable diapers, can absorb up to 60 times its weight in
urine.
the total
concentration of positive and negative ions in the solution. This
polymer can absorb 800 times it own weight of distilled water,
but only 300 times its weight of tap water. Because the ionic
strength of urine is equivalent to an 0.1 M NaCl
solution, this superabsorbant polymer, which can be found in
disposable diapers, can absorb up to 60 times its weight in
urine.

Condensation
Polymers
The first plastic (Celluloid) and the first artificial fiber
(Rayon) were produced from cellulose.
The first truly synthetic plastic was bakelite, developed by Leo
Baekland between 1905 and 1914. The synthesis of bakelite starts
with the reaction between formaldehyde (H2CO) and
phenol (C6H5OH) to form a mixture of ortho-
and para-substituted phenols. At temperatures above
100C, these phenols condense to form a polymer in which the
aromatic rings are bridged by either -CH2OCH2-
or -CH2- linkages. The cross-linking in this polymer
is so extensive that it is a thermoset plastic. Once it is
formed, any attempt to change the shape of this plastic is doomed
to failure.
Research started by Wallace Carothers and coworkers at DuPont
in the 1920s and 1930s eventually led to the discovery of the
families of condensation polymers known as polyamides and
polyesters. The polyamides were obtained by
reacting a diacyl chloride with an diamine.
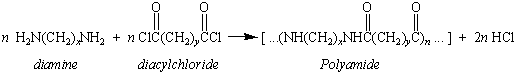
The polyesters were made by reacting the
diacyl chloride with a dialcohol.
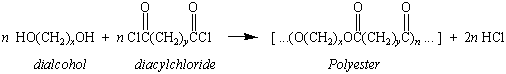
While studying polyesters, Julian Hill found that he could
wind a small amount of this polymer on the end of a stirring rod
and draw it slowly out of solution as a silky fiber. One day,
when Carothers wasn't in the lab, Hill and his colleagues tried
to see how long a fiber they could make by stretching a sample of
this polymer as they ran down the hall. They soon realized that
this playful exercise had oriented the polymer molecules in two
dimensions and produced a new material with superior properties.
They then tried the same thing with one of the polyamides and
produced a sample of what became the first synthetic fiber: Nylon.
This process can be demonstrated by carefully pouring a
solution of hexamethylenediamine in water on top of a solution of
adipoyl chloride in CH2Cl2.
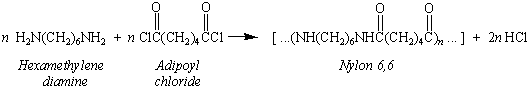
A thin film of polymer forms at the interface between these
two phases. By grasping this film with a pair of tweezers, we can
draw a continuous string of nylon from the solution. The product
of this reaction is known as Nylon 6,6 because the polymer is
formed from a diamine that has six carbon atoms and a derivative
of a dicarboxylic acid that has six carbon atoms.
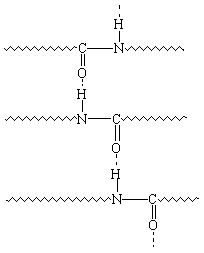
The effect of pulling on the polymer with the tweezers is much
like that of stretching an elastomer  the polymer molecules become
oriented in two dimensions. Why don't the polymer molecules
return to their original shape when we stop pulling? Section P.3 suggested that polymers are elastic
when there is no strong force of attraction between the polymer
chains. Polyamides and polyesters form strong hydrogen bonds
between the polymer chains that keep the polymer molecules
oriented, as shown in the figure below.
the polymer molecules become
oriented in two dimensions. Why don't the polymer molecules
return to their original shape when we stop pulling? Section P.3 suggested that polymers are elastic
when there is no strong force of attraction between the polymer
chains. Polyamides and polyesters form strong hydrogen bonds
between the polymer chains that keep the polymer molecules
oriented, as shown in the figure below.
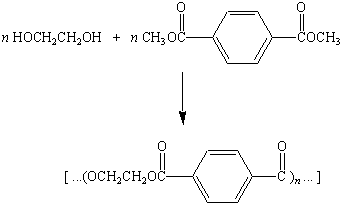
The first polyester fibers were produced by reacting ethylene
glycol and either terephthalic acid or one of its esters to give
poly(ethylene terephthalate). This polymer is still used to make
thin films (Mylar) and textile fibers (Dacron and Fortrel).
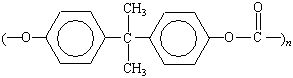
Phosgene (COCl2) reacts with alcohols
to form esters that are analogous to those formed when acyl
chlorides react with alcohols.
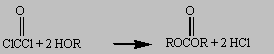
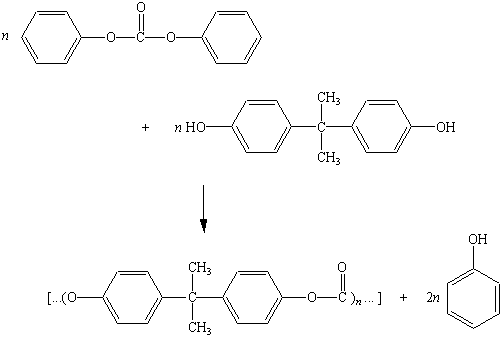
The product of this reaction is called a carbonate ester
because it is the diester of carbonic acid, H2CO3.
Polycarbonates are produced when one of these
esters reacts with an appropriate alcohol, as shown in the figure
below. The polycarbonate shown in this figure is known as Lexan.
It has a very high resistance to impact and is used in safety
glass, bullet-proof windows, and motorcycle helmets.
The structures and names of some common condensation polymers
are given in the table below.
Common Condensation Polymers
| Structure |
|
Trade Name or
Common Name |
| |
|
Polyamides |
|
|
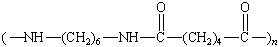 |
|
Nylon 66 |
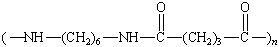 |
|
Nylon 610 |
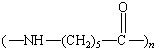 |
|
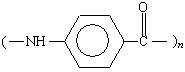
Nylon 6 |
 |
|
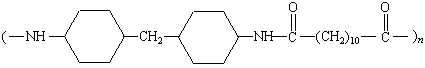
Qiana |
| |
|
Polyaramides |
|
|
 |
|
Kevlar |
| |
|
Polyesters |
|
|
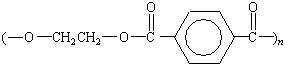 |
|
Dacron, Mylar |
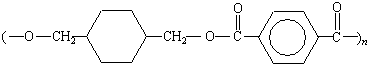 |
|
Kodel |
| |
|
Polycarbonates |
|
|
 |
|
Lexan |
| |
|
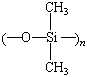
Silicones |
|
|
 |
|
silicone rubber |