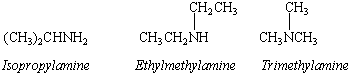
Amines, Alkaloids, and Amides
Amines are derivatives of ammonia in which one or more hydrogen atoms are replaced by alkyl groups. We indicate the degree of substitution by labeling the amine as either primary (RNH2), secondary (R2NH), or tertiary (R3N). The common names of these compounds are derived from the names of the alkyl groups.
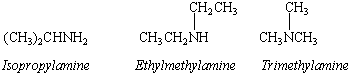
The systematic names of primary amines are derived from the name of the parent alkane by adding the prefix -amino and a number specifying the carbon that carries the -NH2 group.
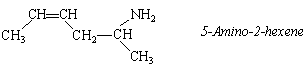
The chemistry of amines mirrors the chemistry of ammonia. Amines are weak bases that pick up a proton to form ammonium salts. Trimethylamine, for example, reacts with acid to form the trimethylammonium ion.

These salts are more soluble in water than the corresponding amines, and this reaction can be used to dissolve otherwise insoluble amines in aqueous solution.
The difference between amines and their ammonium salts plays an important role in both over-the-counter and illicit drugs. Cocaine, for example, is commonly sold as the hydrochloride salt, which is a white, crystalline solid. By extracting this solid into ether, it is possible to obtain the "free base." A glance at the side panel of almost any over-the-counter medicine will provide examples of ammonium salts of amines that are used to ensure that the drug dissolves in water (see figure below).
 |
Dextromethorphan hydrobromide (cough suppressant) |
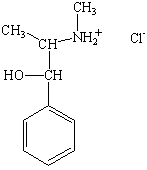 |
Pseudoephedrine hydrochloride (decongestant) |
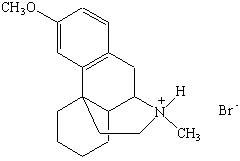
Amines that are isolated from plants are known as alkaloids. They include poisons such as nicotine, coniine, and strychnine (shown in Figure the figure below). Nicotine has a pleasant, invigorating effect when taken in minuscule quantities, but is extremely toxic in larger amounts. Coniine is the active ingredient in hemlock, a poison that has been used since the time of Socrates. Strychnine is another toxic alkaloid that has been a popular poison in murder mysteries.
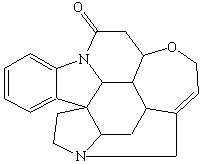 |
Strychnine | ||
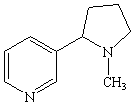 |
Nicotine | 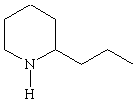 |
Coniine |
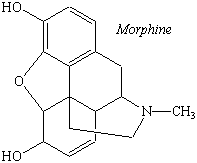
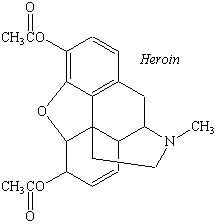
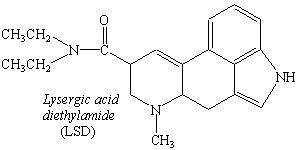
The alkaloids also include a number of drugs, such as morphine, quinine, and cocaine. Morphine is obtained from poppies; quinine can be found in the bark of the chinchona tree; and cocaine is isolated from coca leaves. This family of compounds also includes synthetic analogs of naturally occurring alkaloids, such as heroin and LSD (see figure below).
 |
 |
|
 |
 |
|
 |
||
To illustrate the importance of minor changes in the structure of a molecule, three amines with similar structures are shown in the figure below. One of these compounds is caffeine, which is the pleasantly addictive substance that makes a cup of coffee an important part of the day for so many people. Another is theobromine, which is the pleasantly addictive substance that draws so many people to chocolate. The third compound is theophylline, which is a prescription drug used as a bronchodilator.

It is tempting to assume that carboxylic acids will react with amines to form the class of compounds known as amides. In practice, when aqueous solutions of carboxylic acids and amines are mixed all we get is an acid-base reaction.
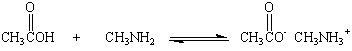
The best way to prepare an amide is to react the appropriate acyl chloride with an amine.
![]()
Excess amine is used to drive the reaction to completion by absorbing the HCl given off in this reaction.