Practice Problem 3
Use half-reactions to balance the equation for the reaction between sulfur dioxide and the dichromate ion in acidic solution.
Solution
STEP 1:Write a skeleton equation for the reaction.
SO2 + Cr2O72-![]() SO42- + Cr3+
SO42- + Cr3+
STEP 2: Assign oxidation numbers to atoms on both sides of the equation.
| SO2 | + Cr2O72- | SO42- | + Cr3+ | ||||
| +4 | -2 | +6 -2 | +6 | -2 | +3 | ||
STEP 3: Determine which atoms are oxidized and which are reduced.
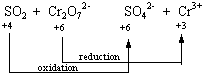
STEP 4: Divide the reaction into oxidation and reduction half-reactions and balance these half-reactions. This reaction can be divided into the following half-reactions.
| Oxidation: | SO2 | SO42- | |||
| +4 | +6 |
| Reduction: | Cr2O72- | Cr3+ | |||
| +6 | +3 |
It doesn't matter which half-reaction we balance first, so let's start with reduction. Because the Cr2O72- ion contains two chromium atoms that must be reduced from the +6 to the +3 oxidation state, six electrons are consumed in this half-reaction.
| Reduction: | Cr2O72- + 6 e- | 2 Cr3+ |
This raises an interesting question: What happens to the oxygen atoms when the chromium atoms are reduced? The seven oxygen atoms in the Cr2O72- ions are formally in the -2 oxidation state. If these atoms were released into solution in the -2 oxidation state when the chromium was reduced, they would be present as O2- ions. But it doesn't make sense to write this half-reaction as follows.
| Reduction: | Cr2O72- + 6 e- | 2 Cr3+ + 7 O2- |
The reaction is being run in an acidic solution and the O2- ion is a very strong base that would immediately react with the H+ ions in this solution to form water, as shown in the figure below.
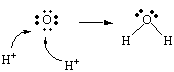
The following is therefore a more realistic equation for this half-reaction.
| Reduction: | Cr2O72- + 14 H+ + 6 e- | 2 Cr3+ + 7 H2O |
We can now turn to the oxidation half-reaction, and start by noting that two electrons are given off when sulfur is oxidized from the +4 to the +6 oxidation state.
| Oxidation: | SO2 | SO42- + 2 e- |
The key to balancing the charge on both sides of this equation is remembering that the reaction is run in acid, which contains H+ ions and H2O molecules. We can therefore add H+ ions or H2O molecules to either side of the equation, as needed. The only way to balance the charge on both sides of this equation is to add four H+ ions to the products of the reaction.
| Oxidation: | SO2 | SO42- + 2 e- + 4 H+ |
We can then balance the number of hydrogen and oxygen atoms on both sides of this equation by adding a pair of H2O molecules to the reactants.
| Oxidation: | SO2 + 2 H2O | SO42- + 2 e- + 4 H+ |
This equation can be understood in terms of the Lewis structures shown in the figure below.
![]()
Let's focus, for the moment, on the middle Lewis structure and remove two electrons from the sulfur atom.
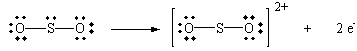
Let's now form two new sulfur-oxygen bonds by donating pairs of non bonding electrons from neighboring water molecules.

The product of this hypothetical reaction can now lose a pair of H+ ions to form sulfurinc acid.

STEP 5: Combine the two half-reactions so that electrons are neither created nor destroyed. Six electrons are consumed in the reduction half-reaction and two electrons are given off in the oxidation half-reaction. We can combine these half-reactions so that electrons are conserved by multiplying the reduction half-reaction by 3.
| (Cr2O72- + 14 H+ + 6 e- |
| + 3(SO2 + 2 H2O |
| __________________________________________ |
| Cr2O72- + 3 SO2 + 14 H+ + 6 H2O
|
STEP 6: Balance the remainder of the equation by inspection, if necessary. Although the equation appears balanced, we are not quite finished with it. We can simplify the equation by subtracting 12 H+ ions and 6 H2O molecules from each side to generate the following balanced equation.
Cr2O72-(aq) + 3 SO2(aq)
+ 2 H+(aq) ![]() 2
Cr3+(aq) + 3 SO42-(aq) + H2O(l)
2
Cr3+(aq) + 3 SO42-(aq) + H2O(l)