Practice Problem 5
An endless number of balanced equations can be written for the reaction between the
permanganate ion and hydrogen peroxide in acidic solution to form the manganese (II) ion
and oxygen:
| MnO4-(aq) |
+ |
H2O2(aq) |
 |
Mn2+(aq) |
+ |
O2(g) |
Use the half-reaction method to determine the correct stoichiometry for this reaction.
Solution
- STEP 1: Write a skeleton equation for the reaction.
MnO4- + H2O2 Mn2+ + O2
Mn2+ + O2
-
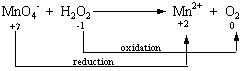
- STEP 2: Assign oxidation numbers to atoms on both sides of the
equation.
| MnO4- |
+ |
H2O2 |
 |
Mn2+ |
+ |
O2 |
| +7 -2 |
|
+1-1 |
|
+2 |
|
0 |
STEP 3: Determine which atoms are oxidized and
which are reduced.
-
- STEP 4: Divide the reaction into oxidation and reduction
half-reactions and balance these half-reactions. We balanced the reduction
half-reaction in the previous exercise.
-
| Reduction: |
|
MnO4- + 8 H+ + 5 e- |
 |
Mn2+ + 4 H2O |
-
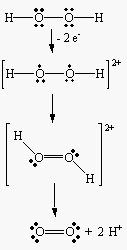
- To balance the oxidation half-reaction, we have to remove two electrons from a pair of
oxygen atoms in the -1 oxidation state to form a neutral O2 molecule.
-
| Oxidation: |
|
H2O2 |
 |
O2 + 2 e- |
-
- We can then add a pair of H+ ions to the products to balance both charge and
mass in this half-reaction
-
| Oxidation: |
|
H2O2 |
 |
O2 + 2 H+ + 2 e- |
-
- as shown in the following figure.
-
- STEP 5: Combine the two half-reactions so that electrons are neither created nor
destroyed. Two electrons are given off during oxidation and five electrons are
consumed during reduction. We can combine these half-reactions so that electrons are
conserved by using the lowest common multiple of 5 and 2.
-
2(MnO4- + 8 H+ + 5 e- Mn2+ + 4 H2O) Mn2+ + 4 H2O) |
+ 5(H2O2 O2 + 2 H+ + 2 e-)
O2 + 2 H+ + 2 e-) |
| ________________________________________________________ |
2 MnO4- + 5 H2O2 + 16 H+ 2 Mn2+ + 5 O2 +
10 H+ + 8 H2O 2 Mn2+ + 5 O2 +
10 H+ + 8 H2O |
-
- STEP 6: Balance the remainder of the equation by inspection, if necessary.
The simplest balanced equation for this reaction is obtained when 10 H+ ions
are subtracted from each side of the equation derived in the previous step.
2 MnO4-(aq) + 5 H2O2(aq)
+ 6 H+(aq) 2
Mn2+(aq) + 5 O2(g) + 8 H2O(l)
2
Mn2+(aq) + 5 O2(g) + 8 H2O(l)


![]() Mn2+ + O2
Mn2+ + O2 
![]() 2
Mn2+(aq) + 5 O2(g) + 8 H2O(l)
2
Mn2+(aq) + 5 O2(g) + 8 H2O(l)