Br2 + OH-![]() Br- + BrO3-
Br- + BrO3-
| Br2 | + | OH- | Br - | + | BrO3- | |
| 0 | -2 +1 | -1 | +5 -2 |
STEP 3: Determine which atoms are oxidized and which are reduced.
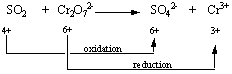
STEP 4: Divide the reaction into oxidation and reduction half-reactions and balance these half-reactions. Bromine is oxidized from the 0 to the +5 oxidation state in this reaction.
| Oxidation: | Br2 | BrO3- | |||
| 0 | +5 |
Bromine is also reduced in this reaction, from the 0 to the -1 oxidation state.
| Reduction: | Br2 | Br- | |||
| 0 | -1 |
If we assume that two Br- ions are produced for each molecule of Br2 reduced, the reduction half-reaction would be written as follows.
| Reduction: | Br2 + 2 e- | 2 Br - |
| Oxidation: | Br2 | 2 BrO3- + 10 e- |
| Oxidation: | Br2 + 12 OH- | 2 BrO3- + 10 e- |
| Oxidation: | Br2 + 12 OH- | 2 BrO3- + 10 e- + 6 H2O |
| 5(Br2 + 2 e- |
| + (Br2 + 12 OH- |
| ___________________________________________ |
| 6 Br2 + 12 OH- |
3 Br2 + 6 OH-![]() 5 Br- + BrO3- + 3 H2O
5 Br- + BrO3- + 3 H2O
3 Br2(aq) + 6 OH-(aq)![]() 5 Br-(aq) + BrO3-(aq)
+ 3 H2O(l)
5 Br-(aq) + BrO3-(aq)
+ 3 H2O(l)