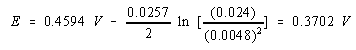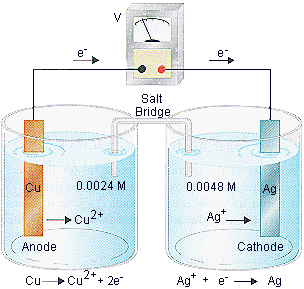
Practice Problem 9
Calculate the potential at 25oC for the following cell.
| Cu | | | Cu2+(0.024 M) | || | Ag+(0.0048 M) | | | Ag |
Solution

We start by translating the line notation into an equation for the reaction.
Cu(s) + 2 Ag+(aq)
Cu2+(aq) + 2 Ag(s)
We then look up the standard-state potentials for the two half-reactions and calculate the standard-state cell potential.
| Oxidation: | Cu |
Eoox = -(0.3402 V) | ||
| Reduction: | Ag+ + e- |
Eored = 0.7996 V | ||
| ŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻ | ||||
| Eo = Eored + Eoox = 0.4594 V |
We now set up the Nernst equation for this cell, noting that n is 2 because two electrons are transferred in the balanced equation for the reaction.
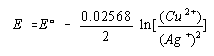
Substituting the concentrations of the Cu2+ and Ag+ ions into this equation gives the value for the cell potential.