| Zn(NH3)42+
+ 2 e- |
Eored = -1.04 V | ||
| Zn2+ + 2 e- |
Eored = -0.7628 V |
Practice Problem 11
Use the following standard-state cell potentials to calculate the complex formation equilibrium constant for the Zn(NH3)42+ complex ion.
| Zn(NH3)42+
+ 2 e- |
Eored = -1.04 V | ||
| Zn2+ + 2 e- |
Eored = -0.7628 V |
Solution
By reversing the first half-reaction and adding it to the second, we can obtain an overall equation that corresponds to the complex formation equilibrium.
| Eoox = 1.04 V | |||
| + Zn2+ + |
Eored = -0.7628 V | ||
| ŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻ | ŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻ | ||
| Zn2+ + 4 NH3 |
Eo = 0.28 V |
When this reaction comes to equilibrium, the cell potential is zero and the reaction quotient is equal to the complex formation equilibrium constant for the Zn(NH3)42+ complex ion.
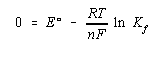
We now solve this equation for the natural logarithm of the equilibrium constant and calculate the value of Kf for this reaction.
![]()
![]()