| Mg(OH)2 + 2 e- |
Eored = -2.69 V | ||
| Mg2+ + 2 e- |
Eored = -2.375 V |
Practice Problem 12
Use the following standard-state cell potentials to calculate the solubility product at 25oC for Mg(OH)2.
| Mg(OH)2 + 2 e- |
Eored = -2.69 V | ||
| Mg2+ + 2 e- |
Eored = -2.375 V |
Solution
This time we have to reverse the second half-reaction to obtain an overall equation that corresponds to the appropriate equilibrium expression.
| Mg(OH)2 + |
Eored = -2.69 V | ||
| + |
Eoox = 2.375 V | ||
| ŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻŻ | ŻŻŻŻŻŻŻŻŻŻŻŻŻ | ||
| Mg(OH)2 |
Eo = -0.32 V |
Once again, we start with the Nernst equation and assume that the reaction is at equilibrium.
![]()
We then rearrange this equation, substitute what we know about the reaction into this equation, and calculate the value of Ksp for this compound.
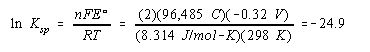
![]()