Practice Problem 10
Use the following standard-state free energy of formation data to calculate the acid-dissociation equilibrium constant (Ka) at for formic acid:
Compound
![]() Gfo(kJ/mol)
Gfo(kJ/mol)
HCO2(aq) -372.3
H+(aq) 0.00
HCO2-(aq) -351.0
Solution
We can start by writing the equation that corresponds to the acid-dissociation equilibrium for formic acid:
HCO2H(aq) ![]() H+(aq) +
HCO2-(aq)
H+(aq) +
HCO2-(aq)
We then calculate the value of ![]() Go
for this reaction:
Go
for this reaction:
![]() Go
=
Go
= ![]() Gfo(products)
-
Gfo(products)
- ![]() Gfo(reactants)
Gfo(reactants)
= [1 mol H+ x 0.00 kJ/mol + 1 mol HCO2- x -351.0 kJ/mol] - [1 mol HCO2H x -372.3 kJ/mol]
= 21.3 kJ
We now turn to the relationship between ![]() Go and the equilibrium constant for the
reaction:
Go and the equilibrium constant for the
reaction:
![]() Go
= - RT ln K
Go
= - RT ln K
and solve for the natural log of the equilibrium constant:
![]()
Substituting the known value of ![]() Go, R, and T into this
equation gives the following result:
Go, R, and T into this
equation gives the following result:
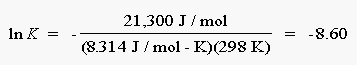
We can now calculate the value of the equilibrium constant:
K = e-8.60 = 1.8 x10-4
The value of Ka obtained from this calculation agrees with the table value for formic acid, within experimental error.