Practice Problem 3
Use the Lewis structures of NO2 and N2O4 and the
stoichiometry of the following reaction to decide whether ![]() H and
H and ![]() S favor the reactants or products of this reaction:
S favor the reactants or products of this reaction:
2 NO2(g) ![]() N2O4(g)
N2O4(g)
Solution
NO2 has an unpaired electron in its Lewis structure:
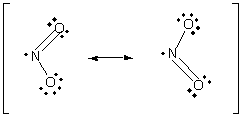
When two NO2 molecules collide in the proper orientation, a covalent bond can form between the nbitrogen atoms to produce the dimer, N2O4:
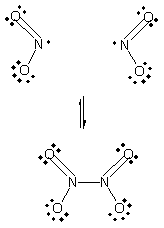
Because it forms a new bond, this reaction should be exothermic. ![]() H for this reaction
therefore favors the products. The reaction leads to a decrease in the entropy of
the system because the system becomes less disordered when two molecules come together to
form a larger molecule
H for this reaction
therefore favors the products. The reaction leads to a decrease in the entropy of
the system because the system becomes less disordered when two molecules come together to
form a larger molecule ![]() S for this reaction therefore favors the reactants.
S for this reaction therefore favors the reactants.