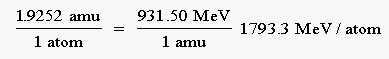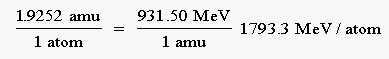
Practice Problem 5
Calculate the binding energy of 235U if the mass of this nuclide is 235.0349 amu.
Solution
A neutral 235U atom contains 92 protons, 92 electrons, and 143 neutrons. The predicted mass of a 235U atom is therefore 236.9601 amu, to four significant figures:
[92(1.00728) amu] + [92(0.0005486) amu] + [143(1.00867) amu] = 236.9601 amu
total mass = 236.9601 amu
To calculate the mass defect for this nuclide, we subtract the observed mass from the predicted mass:
236.9601 amu - 235.0349 amu = 1.9252 amu
mass defect = 1.9252 amu
Using the conversion factor that relates the binding energy to the mass defect, we obtain a binding energy for 235U of 1793.3 MeV per atom: