The Inorganic Chemistry of Carbon
For more than 200 years, chemists have divided compounds into two categories. Those that were isolated from plants or animals were called organic, while those extracted from ores and minerals were inorganic. Organic chemistry is often defined as the chemistry of carbon. But this definition would include calcium carbonate (CaCO3) and graphite, which more closely resemble inorganic compounds. We will therefore define organic chemistry as the study of compounds, such as formic acid (HCO2H), methane (CH4), and vitamin C (C6H8O6), that contain both carbon and hydrogen.
The chemistry of carbon is dominated by three factors.
1. Carbon forms unusually strong C-C single bonds, C=C double bonds, and carbon-carbon triple bonds.
2. The electronegativity of carbon (EN = 2.55) is too small to allow carbon to form C4- ions with most metals and too large for carbon to form C4+ ions when it reacts with nonmetals. Carbon therefore forms covalent bonds with many other elements.
3. Carbon forms strong double and triple bonds with a number of other nonmetals, including N, O, P, and S.
Elemental Forms of Carbon: Graphite, Diamond, Coke, and Carbon Black
Carbon occurs as a variety of allotropes. There are two
crystalline forms ![]() diamond and graphite
diamond and graphite![]() and a number of amorphous
(noncrystalline) forms, such as charcoal, coke, and carbon black.
and a number of amorphous
(noncrystalline) forms, such as charcoal, coke, and carbon black.
References to the characteristic hardness of diamond (from the Greek adamas, "invincible") date back at least 2600 years. It was not until 1797, however, that Smithson Tennant was able to show that diamonds consist solely of carbon. The properties of diamond are remarkable. It is among the least volatile substances known (MP = 3550oC, BP = 4827oC), it is also the hardest substance known, and it expands less on heating than any other material.
The properties of diamond are a logical consequence of its structure. Carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in the figure below. Each of these sp3-hybridized atoms is then bound to four other carbon atoms, which form bonds to four other carbon atoms, and so on. As a result, a perfect diamond can be thought of as a single giant molecule. The strength of the individual C-C bonds and their arrangement in space give rise to the unusual properties of diamond.
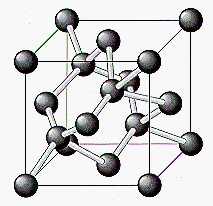
In some ways, the properties of graphite are like those of diamond. Both compounds boil at 4827oC, for example. But graphite is also very different from diamond. Diamond (3.514 g/cm3) is much denser than graphite (2.26 g/cm3). Whereas diamond is the hardest substance known, graphite is one of the softest. Diamond is an excellent insulator, with little or no tendency to carry an electric current. Graphite is such a good conductor of electricity that graphite electrodes are used in electrical cells.
The physical properties of graphite can be understood from the structure of the solid shown in the figure below.
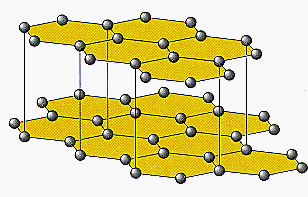
Graphite consists of extended planes of sp2-hybridized carbon atoms in which each carbon is tightly bound to three other carbon atoms. (The strong bonds between carbon atoms within each plane explain the exceptionally high melting point and boiling point of graphite.) The distance between these planes of atoms, however, is very much larger than the distance between the atoms within the planes. Because the bonds between planes are weak, it is easy to deform the solid by allowing one plane of atoms to move relative to another. As a result, graphite is soft enough to be used in pencils and as a lubricant in motor oil.
"Lead" pencils do not, incidentally, contain lead. (This is fortunate because many people chew pencils and lead compounds are toxic.) Lead pencils contain graphite, or "black lead" as it was once known, which is mixed with clay (20% to 60% by weight) and then baked to form a ceramic rod. Increasing the percentage of clay makes the pencil harder, so that less graphite is deposited on the paper.
The characteristic properties of graphite and diamond might
lead you to expect that diamond would be more stable than
graphite. This is not what is observed experimentally. The
standard enthalpy of formation of diamond (![]() Hof
= 2.425 kJ/mol) is slightly larger than the enthalpy of formation
of graphite, which is the most stable form of carbon at 25oC
and 1 atm pressure. At very high temperatures and pressures,
diamond becomes more stable than graphite. In 1955 General
Electric developed a process to make industrial-grade diamonds by
treating graphite with a metal catalyst at temperatures of 2000
to 3000 K and pressures above 125,000 atm. Roughly 40% of
industrial-quality diamonds are now synthetic. Although
gem-quality diamonds can be synthesized, the costs involved are
prohibitive.
Hof
= 2.425 kJ/mol) is slightly larger than the enthalpy of formation
of graphite, which is the most stable form of carbon at 25oC
and 1 atm pressure. At very high temperatures and pressures,
diamond becomes more stable than graphite. In 1955 General
Electric developed a process to make industrial-grade diamonds by
treating graphite with a metal catalyst at temperatures of 2000
to 3000 K and pressures above 125,000 atm. Roughly 40% of
industrial-quality diamonds are now synthetic. Although
gem-quality diamonds can be synthesized, the costs involved are
prohibitive.
Both diamond and graphite occur as regularly packed crystals.
Other forms of carbon are amorphous ![]() they lack a
regular structure. Charcoal, carbon black, and coke are all
amorphous forms of carbon. Charcoal results from heating
wood in the absence of oxygen. To make carbon black,
natural gas or other carbon compounds are burned in a limited
amount of air to give a thick, black smoke that contains
extremely small particles of carbon, which can be collected when
the gas is cooled and passed through an electrostatic
precipitator. Coke is a more regularly structured
material, closer in structure to graphite than either charcoal or
carbon black, which is made from coal.
they lack a
regular structure. Charcoal, carbon black, and coke are all
amorphous forms of carbon. Charcoal results from heating
wood in the absence of oxygen. To make carbon black,
natural gas or other carbon compounds are burned in a limited
amount of air to give a thick, black smoke that contains
extremely small particles of carbon, which can be collected when
the gas is cooled and passed through an electrostatic
precipitator. Coke is a more regularly structured
material, closer in structure to graphite than either charcoal or
carbon black, which is made from coal.
Carbides: Covalent, Ionic, and Interstitial
Although carbon is essentially inert at room temperature, it reacts with less electronegative negative elements at high temperatures to form compounds known as carbides. When carbon reacts with an element of similar size and electronegativity, a covalent carbide is produced. Silicon carbide, for example, is made by treating silicon dioxide from quartz with an excess of carbon in an electric furnace at 2300 K.
| SiO2(s) | + | 3 C(s) | SiC(s) | + | 2 CO(g) |
Covalent carbides have properties similar to those of diamond. Both SiC and diamond are inert to chemical reactions, except at very high temperatures; both have very high melting points; and both are among the hardest substances known. SiC was first synthesized by Edward Acheson in 1891. Shortly thereafter, Acheson founded the Carborundum Company to market this material. Then, as now, materials in this class are most commonly used as abrasives.
Compounds that contain carbon and one of the more active metals are called ionic carbides.
| CaO(s) | + | 3 C(s) | CaC2(s) | + | CO(g) |
It is useful to think about these compounds as if they contained negatively charged carbon ions: [Ca2+][C22-] or [Al3+]4[C4-]3. This model is useful because it explains why these carbides burst into flame when added to water. The ionic carbides that formally contain the C4- ion react with water to form methane, which is ignited by the heat given off in this reaction.
| C4- | + | 4 H2O | CH4 | + | 4 OH- |
The ionic carbides that formally contain the C22- ion react with water to form acetylene, which is ignited by the heat of reaction.
| C22- | + | 2 H2O | C2H2 | + | 2 OH- |
At one time, miners' lamps were fueled by the combustion of acetylene prepared from the reaction of calcium carbide with water.
Interstitial carbides, such as tungsten carbide (WC), form when carbon combines with a metal that has an intermediate electronegativity and a relatively large atomic radius. In these compounds, the carbon atoms pack in the holes (interstices) between planes of metal atoms. The interstitial carbides, which include TiC, ZrC, and MoC retain the properties of metals. They act as alloys, rather than as either salts or covalent compounds.
Although the different forms of carbon are essentially inert at room temperature, they combine with oxygen at high temperatures to produce a mixture of carbon monoxide and carbon dioxide.
| 2 C(s) | + | O2(g) | 2 CO(g) | |||||
| C(s) | + | O2(g) | CO2(g) |
CO can also be obtained when red-hot carbon is treated with steam.
| C(s) | + | H2O(g) | CO(g) | + | H2(g) |
Because this mixture of gases is formed by the reaction of charcoal or coke with water it is often referred to as water gas. It is also known as town gas because it was once made by towns and cities for use as a fuel. Water gas, or town gas, was a common fuel for both home and industrial use before natural gas became readily available. The H2 burns to form water, and the CO is oxidized to CO2. Eventually, as our supply of natural gas is depleted, it will become economical to replace natural gas with other fuels, such as water gas, that can be produced from our abundant supply of coal.
CO and CO2 are both colorless gases. CO boils at -191.5oC, and CO2 sublimes at -78.5oC. Although CO has no odor or taste, CO2 has a faint, pungent odor and a distinctly acidic taste. Both are dangerous substances but at very different levels of exposure. Air contaminated with as little as 0.002 grams of CO per liter can be fatal because CO binds tightly to the hemoglobin and myoglobin that carry oxygen through the blood. CO2 is not lethal until the concentration in the air approaches 15%. At that point, it has replaced so much oxygen that a person who attempts to breathe this atmosphere suffocates. The danger of CO2 poisoning is magnified by the fact that CO2 is roughly 1.5 times more dense than the air in our atmosphere. Thus, CO2 can accumulate at the bottom of tanks or wells.
CO2 influences the temperature of the atmosphere through the greenhouse effect, which works as follows. CO2 absorbs some of the lower energy, longer wavelength infrared radiation from the sun that would otherwise be reflected back from the surface of the planet. Thus, CO2 in the atmosphere traps heat. Although there are other factors at work, it is worth noting that Venus, whose atmosphere contains a great deal of CO2, has a surface temperature of roughly 400oC, whereas Mars, with little or no atmosphere, has a surface temperature of -50oC.
There are many sources of CO2 in the atmosphere. Over geologic time scales, the largest source has been volcanoes. Within the last century, the combustion of petroleum, coal, and natural gas has made a significant contribution to atmospheric levels of CO2 (see figure below). Between 1958 and 1978, the average level of CO2 in the atmosphere increased by 6%, from 315.8 to 334.6 ppm.
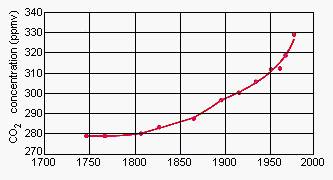
At one time, the amount of CO2 released to the atmosphere was not a matter for concern because natural processes that removed CO2 from the atmosphere could compensate for the CO2 that entered the atmosphere. The vast majority of the CO2 liberated by volcanic action, for example, was captured by calcium oxide or magnesium oxide to form calcium carbonate or magnesium carbonate.
| CaO(s) | + | CO2(g) | CaCO3(s) | |
| MgO(s) | + | CO2(g) | MgCO3(s) |
CaCO3 is found as limestone or marble, or mixed with MgCO3 as dolomite. The amount of CO2 in deposits of carbonate minerals is at least several thousand times larger than the amount in the atmosphere.
CO2 also dissolves, to some extent, in water.
| H2O | |||
| CO2(g) | CO2(aq) |
It then reacts with water to form carbonic acid, H2CO3.
| CO2(aq) | + | H2O(l) | H2CO3(aq) |
As a result of these reactions, the sea contains about 60 times more CO2 than the atmosphere.
Can the sea absorb more CO2 from the atmosphere, or is it near its level of saturation? Is the rate at which the sea absorbs CO2 greater than the rate at which we are adding it to the atmosphere? The observed increase in the concentration of CO2 in recent years suggests pessimistic answers to these two questions. A gradual warming of the earth's atmosphere could result from continued increases in CO2 levels, with adverse effects on the climate and therefore the agriculture of at least the northern hemisphere.
The Chemistry of Carbonates: CO32- and HCO3-
Egg shells are almost pure calcium carbonate. CaCO3 can also be found in the shells of many marine organisms and in both limestone and marble. The fact that none of these substances dissolve in water suggests that CaCO3 is normally insoluble in water. Calcium carbonate will dissolve in water saturated with CO2, however, because carbonated water (or carbonic acid) reacts with calcium carbonate to form calcium bicarbonate, which is soluble in water.
| CaCO3(s) | + | H2CO3(aq) | Ca2+(aq) | + | 2 HCO3-(aq) |
When water rich in carbon dioxide flows through limestone formations, part of the limestone dissolves. If the CO2 escapes from this water, or if some of the water evaporates, solid CaCO3 is redeposited. When this happens as water runs across the roof of a cavern, stalactites, which hang from the roof of the cave, are formed. If the water drops before the carbonate reprecipitates, stalagmites, which grow from the floor of the cave, are formed.
The chemistry of carbon dioxide dissolved in water is the basis of the soft drink industry. The first artificially carbonated beverages were introduced in Europe at the end of the nineteenth century. Carbonated soft drinks today consist of carbonated water, a sweetening agent (such as sugar, saccharin, or aspartame), an acid to impart a sour or tart taste, flavoring agents, coloring agents, and preservatives. As much as 3.5 liters of gaseous CO2 dissolve in a liter of soft drink. The CO2 contributes the characteristic bite associated with carbonated beverages.
Carbonate chemistry plays an important role in other parts of the food industry as well. Baking soda, or bicarbonate of soda, is sodium bicarbonate, NaHCO3, a weak base, which is added to recipes to neutralize the acidity of other ingredients. Baking powder is a mixture of baking soda and a weak acid, such as tartaric acid or calcium hydrogen phosphate (CaHPO4). When mixed with water, the acid reacts with the HCO3- ion to form CO2 gas, which causes the dough or batter to rise.
| HCO3-(aq) | + | H+(aq) | H2CO3(aq) | H2O(l) | + | CO2(g) |
Before commercial baking powders were available, cooks obtained the same effect by mixing roughly a teaspoon of baking soda with a cup of sour milk or buttermilk. The acids that give sour milk and buttermilk their characteristic taste also react with the bicarbonate ion to give CO2.
In 1985 Richard Smalley and co-workers at Rice University made a uniquely stable form of carbon by vaporizing graphite with a laser. The apparatus in which this experiment was performed was designed to create small molecules that were clusters of atoms. In this cluster generator, a pulse of helium gas was swept over the surface of the graphite as it was excited with the laser. The mixture of helium and carbon atoms that vaporized from the graphite surface cooled as the gas expanded, and molecules with the formula C60 were formed that have a structure that has the symmetry of a soccer-ball. Because this structure resembles the geodesic dome invented by R. Buckminster Fuller, C60 was named buckminsterfullerene, or "buckyball" for short.
Although it was formally a new form of pure carbon, C60 seemed to be nothing more than a laboratory curiosity until 1990, when Wolfgang Kratschmer and Konsantinos Kostiropoulos, at the Max Planck Institute in Heidelberg, reported that this material could be made by heating a graphite rod in an atmosphere of helium until the graphite evaporated. Once it was known that C60 could be synthesized in large quantities, researchers looked for, and found it, in such common sources as the flame of a sooty candle. It has even been found in the black soot that collects on the glass screen in front of a fireplace.
Some of the excitement chemists experienced when C60 was synthesized can be understood by contrasting this form of pure carbon with diamond and graphite. C60 is unique because it exists as distinct molecules, not extended arrays of atoms. Equally important, C60 can be obtained as a pure substance, whereas the surfaces of diamond and graphite are inevitably contaminated by hydrogen atoms that bind to the carbon atoms on the surface.
C60 is now known to be a member of a family of compounds known as the fullerenes. Other compounds in this family include C32, C44, C50, C58, and C70. C60
may be the most important of the fullerenes because it is the most perfectly symmetric molecule possible, spinning in the solid at a rate of more than 100 million times per second. Because of their symmetry, C60 molecules pack as regularly as Ping-Pong balls. The resulting solid has unusual properties. Initially, it is as soft as graphite, but when compressed by 30%, it becomes harder than diamond. When this pressure is released, the solid springs back to its original volume. C60 therefore has the remarkable property that it bounces back when shot at a metal surface at high speeds.
C60 also has the remarkable ability to form
compounds in which it is an insulator, a conductor, a
semiconductor, or a superconductor. By itself, C60 is
a semiconductor. When mixed with just enough potassium to give a
compound with the empirical formula K3C60,
it conducts electricity like a metal. When excess potassium is
added, this solid becomes an insulator. When K3C60
is cooled to 18 K, the result is a superconductor. The potential
of fullerene chemistry for both practical materials and
laboratory curiosities is large enough to explain why this
molecule can be described as "exocharmic" ![]() it exudes
charm.
it exudes
charm.
