Hydrocarbons
| Naturally Occurring Hydrocarbons and Their Derivatives | Aromatic Hydrocarbons and Their Derivatives | Consequences of The Spin of Subatomic Particles |
Naturally Occurrig Hydrocarbons and their Derivatives
Complex hydrocarbons and their derivatives are found throughout nature. Natural rubber,
for example, is a hydrocarbon that contains long chains of alternating C=C double bonds
and C![]() C single bonds.
C single bonds.

Writing the structure of complex hydrocarbons can be simplified by using a line notation in which a carbon atom is assumed to be present wherever a pair of lines intersect and enough hydrogen atoms are present to satisfy the tendency of carbon to form a total of four bonds.

There are a variety of techniques for isolating both pleasant and foul-smelling compounds known as essential oils from natural sources, particularly from plants. These compounds are not "essential," in the sense of being vital to life. They were given that name because they give off a distinct "essence," or smell.
The essential oils are used in perfumes and medicines. Some of these compounds can be isolated by gently heating, or steam distilling, the crushed flowers of plants. Others can be extracted into nonpolar solvents, or absorbed onto grease-coated cloths in which the plants are wrapped. Many of these essential oils belong to classes of compounds known as terpenes and terpenoids. The terpenes are hydrocarbons that usually contain one or more C=C double bonds. The terpenoids are oxygen-containing analogs of the terpenes.
Examples of terpenes include -pinene and -pinene, the primary components of turpentine that give rise to its characteristic odor.

Camphor and menthol are examples of terpenoids.

Both of these compounds have a fragrant, penetrating odor and taste cool. Camphor is used as a moth repellent. Menthol is a mild anesthetic that is added to some brands of cigarettes. The terpenoids also include compounds such as geranial and neral, a pair of cis/trans stereoisomers that can be found in lemon oil. Geranial has a strong lemon odor. Neral tastes sweeter, but has a less intense odor.

Although the terpenes and terpenoids discussed so far have very different structures, they have one important property in common: They all contain 10 carbon atoms, neither more nor less. Each of these compounds can be traced back to a reaction in which a pair of five-carbon molecules are fused. Thus, it isn't surprising that we can also find sesquiterpenes (15 carbon atoms), diterpenes (20 carbon), triterpenes (30 carbons), and so on. Important examples of these compounds include Vitamin A and the b-carotene that gives carrots their characteristic color.


Steroids aren't terpenes or terpenoids in the literal sense because they don't contain the characteristic number of carbon atoms. Consider cholesterol, for example, which is one of the most important steroids.

Analysis of this structure suggests the formula C27H46O, which doesn't fit the pattern expected of a terpenoid. The biosynthetic precursor of this molecule, however, is a 30-carbon triterpene that is converted into cholesterol by a series of enzyme-catalyzed reactions.
By definition, the steroids are compounds that have the basic structure formed by fusing three six-membered rings and a five-membered ring. The most important property of this molecule is the fact that, with the exception of the -OH group on the lower-left-hand corner of the molecule, there is nothing about the structure of this compound that would make it soluble in water.
In this day of cholesterol-free products, it is useful to recognize that it is absurd to label products such as peanut butter as cholesterol-free. That is like saying that the Sahara desert is rain-free. Peanut butter is made from peanuts and cholesterol isn't a characteristic ingredient in plants; it is synthesized by animals, particularly mammals. It is also useful to note that placing someone on a cholesterol-free diet won't reduce their cholesterol level to zero. Even on a low-cholesterol diet, the individual will synthesize about 0.80 grams of cholesterol per day. The key question is: Is there excess of cholesterol in the blood stream? If there is, a diet that reduces the intake of cholesterol might be important.
Cholesterol is the biosynthetic precursor for the synthesis of all of the major classes of hormones, the chemical messengers that coordinate the activity of different cells in a multicellular organism. The steroid hormones include the progestogens, estrogens, and androgens.
Progesterone is an example of a progestogen. This hormone plays a vital role in pregnancy. After ovulation, the corpus luteum secretes progesterone, which prepares the lining of the uterus for implantation of the fertilized ovum. Progesterone is then released by the placenta throughout pregnancy to suppress ovulation. Progesterone was therefore the model on which the first oral contraceptives were built. Progesterone itself is not a good oral contraceptive because this hormone is degraded in the digestive system. It therefore requires massive doses of progesterone to prevent pregnancy when this hormone is taken orally.

The estrogen hormones, such as estrone and estradiol serve three functions. First, they are responsible for the development of the secondary sex characteristics that appear at the onset of puberty in women. Second, they participate in both the ovarian and estrus cycles, and are therefore another model for the design of oral contraceptives. Third, they stimulate the development of the mammary glands during pregnancy.
 |
 |
The androgen hormones, such as androsterone and testosterone play an equivalent role in men, where they are responsible for the secondary sex characteristics that appear at puberty. Testosterone is the true male sex hormone; androsterone is a metabolized form of this steroid that is excreted in the urine.
 |
 |
The Aromatic Hydrocarbons and Their Derivatives
At the turn of the 19th century, one of the signs of living the good life was having gas lines connected to your house, so that you could use gas lanterns to light the house after dark. The gas burned in these lanterns was called coal gas because it was produced by heating coal in the absence of air. The principal component of coal gas was methane, CH4.
In 1825, Michael Faraday was asked to analyze an oily liquid with a distinct odor that collected in tanks used to store coal gas at high pressures. Faraday found that this compound had the empirical formula: CH. Ten years later, Eilhardt Mitscherlich produced the same material by heating benzoic acid with lime. Mitscherlich named this substance benzin, which became benzene when translated into English. He also determined that the molecular formula of this compound is C6H6.
Benzene is obviously an unsaturated hydrocarbon because it has far less hydrogen than the equivalent saturated hydrocarbon: C6H14. But benzene is too stable to be an alkene or alkyne. Alkenes and alkynes rapidly add Br2 to the C=C or CC bonds, whereas benzene only reacts with bromine in the presence of a catalyst: FeBr3. Furthermore, when benzene reacts with Br2 in the presence of FeBr3, the product of this reaction is a compound in which a bromine atom has been substituted for a hydrogen atom, not added to the compound the way an alkene adds bromine.
| FeBr3 | ||
| C6H6 + Br2 | C6H5Br + HBr |
Other compounds were eventually isolated from coal that had similar properties. Their formulas suggested the presence of multiple C=C bonds, but these compounds were not reactive enough to be alkenes. Because they often had a distinct odor, or aroma, they became known as aromatic compounds.
The structure of benzene was a recurring problem throughout most of the 19th century.
The first step toward solving this problem was taken by Friedrich August Kekule in 1865.
(Kekule's interest in the structure of organic compounds may have resulted from the fact
that he first enrolled at the University of Giessen as a student of architecture.) One
day, while dozing before a fire, Kekule dreamed of long rows of atoms twisting in a
snakelike motion until one of the snakes seized hold of its own tail. This dream led
Kekule to propose that benzene consists of a ring of six carbon atoms with alternating C![]() C single bonds and C=C double bonds. Because
there are two ways in which these bonds can alternate, Kekule proposed that benzene was a
mixture of two compounds in equilibrium.
C single bonds and C=C double bonds. Because
there are two ways in which these bonds can alternate, Kekule proposed that benzene was a
mixture of two compounds in equilibrium.

Kekule's structure explained the formula of benzene, but it did not explain why benzene failed to behave like an alkene. The unusual stability of benzene wasn't understood until the development of the theory of resonance. This theory states that molecules for which two or more satisfactory Lewis structures can be drawn are an average, or hybrid, of these structures. Benzene, for example, is a resonance hybrid of the two Kekule structures.

The difference between the equilibrium and resonance descriptions of benzene is subtle, but important. In the equilibrium approach, a pair of arrows is used to describe a reversible reaction, in which the molecule on the left is converted into the one on the right, and vice versa. In the resonance approach, a double-headed arrow is used to suggest that a benzene molecule does not shift back and forth between two different structures; it is a hybrid mixture of these structures.
One way to probe the difference between Kekule's idea of an equilibrium between two
structures and the resonance theory in which benzene is a hybrid mixture of these
structures would be to study the lengths of the carbon-carbon bonds in benzene. If
Kekule's idea was correct, we would expect to find a molecule in which the bonds alternate
between relatively long C![]() C single bonds
(0.154 nm) and significantly shorter C=C double bonds (0.133 nm). When benzene is cooled
until it crystallizes, and the structure of the molecule is studied by x-ray diffraction,
we find that the six carbon-carbon bonds in this molecule are the same length (0.1395 nm).
The crystal structure of benzene is therefore more consistent with the resonance model of
bonding in benzene than the original Kekule structures.
C single bonds
(0.154 nm) and significantly shorter C=C double bonds (0.133 nm). When benzene is cooled
until it crystallizes, and the structure of the molecule is studied by x-ray diffraction,
we find that the six carbon-carbon bonds in this molecule are the same length (0.1395 nm).
The crystal structure of benzene is therefore more consistent with the resonance model of
bonding in benzene than the original Kekule structures.
The resonance theory does more than explain the structure of benzene ![]() it also explains why benzene is less reactive
than an alkene. The resonance theory assumes that molecules that are hybrids of two or
more Lewis structures are more stable than those that aren't. It is this extra stability
that makes benzene and other aromatic derivatives less reactive than normal alkenes. To
emphasize the difference between benzene and a simple alkene, many chemists replace the
Kekule structures for benzene and its derivatives with an aromatic ring in which the
circle in the center of the ring indicates that the electrons in the ring are delocalized;
they are free to move around the ring.
it also explains why benzene is less reactive
than an alkene. The resonance theory assumes that molecules that are hybrids of two or
more Lewis structures are more stable than those that aren't. It is this extra stability
that makes benzene and other aromatic derivatives less reactive than normal alkenes. To
emphasize the difference between benzene and a simple alkene, many chemists replace the
Kekule structures for benzene and its derivatives with an aromatic ring in which the
circle in the center of the ring indicates that the electrons in the ring are delocalized;
they are free to move around the ring.

The significance of the circle in the center of this aromatic ring might best be understood by asking: What is wrong with the Kekule structures for benzene? We start by building a sigma-bond skeleton for the benzene ring in which each of the carbon atoms is sp2 hybridized. This leaves us with one electron in a 2p orbital on each of the six carbon atoms.

If we assume that the interaction between the 2p orbitals is localized between a pair of carbon atoms, we get one of the Kekule structures for benzene. Switching the pairs of atoms that form bonds gives us the other Kekule structure.

But if we allow the six electrons in the six 2p orbitals to interact to form a set of molecular orbitals, we can delocalize the electrons so that they are free to move from one carbon atom to another around the ring.

It is this delocalization of electrons around the aromatic ring that is conveyed by the circle that is often written inside the ring. It is also the delocalization of electrons that makes benzene less reactive than a simple alkene.
Aromatic compounds were being extracted from coal tar as early as the 1830s. As a result, many of these compounds were given common names that are still in use today. A few of these compounds are shown below.

There are three ways in which a pair of substituents can be placed on an aromatic ring. In the ortho (o) isomer, the substituents are in adjacent positions on the ring. In the meta (m) isomer, they are separated by one carbon atom. In the para (p) isomer, they are on opposite ends of the ring. The three isomers of dimethylbenzene, or xylene, are shown below.

|
Predict the structure of para-dichlorobenzene, one of the active ingredients in moth balls. |
Aromatic compounds can contain more than one six-membered ring. Naphthalene, anthracene, and phenanthrene are examples of aromatic compounds that contain two or more fused benzene rings.
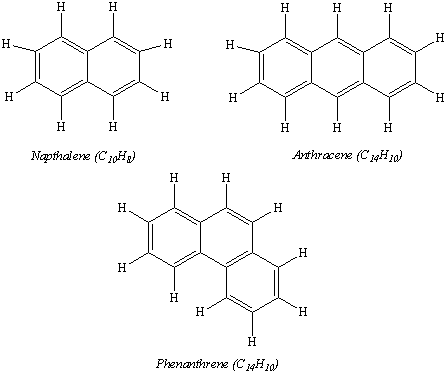
A ball-and-stick model of anthracene is shown in the figure below.

Consequences of The Spin of Subatomic Particles
Electrons aren't the only subatomic particles that behave as if they were spinning in
one direction or another ![]() protons and
neutrons also have a spin quantum number of . Because they contain protons
and neutrons, many (but not all) nuclei also have a spin quantum number.
protons and
neutrons also have a spin quantum number of . Because they contain protons
and neutrons, many (but not all) nuclei also have a spin quantum number.
The table below lists the spin quantum number of the common isotopes of the elements in the first and second rows of the periodic table. Several patterns can be seen in these data.
The Common Isotopes of the First and Second Row Elements
| Nuclei Spin | Quantum Number | Abundance (%) | ||
| 1H (1p) | 99.985 | |||
| 2H (1p, 1n) | 1 | 0.015 | ||
| 4He (2p, 2n) | 0 | |||
| 7Li (3p, 4n) | 3/2 | 92.5 | ||
| 9Be (4p, 5n) | 3/2 | |||
| 10B (5p, 5n) | 3 | 19.9 | ||
| 11B (5p, 6n) | 3/2 | 80.1 | ||
| 12C (6p, 6n) | 0 | 98.90 | ||
| 13C (6p, 7n) | 1.10 | |||
| 14N (7p, 7n) | 1 | 99.63 | ||
| 16O (8p, 8n) | 0 | 99.76 | ||
| 19F (9p, 10n) | ||||
| 20Ne (10p, 10n) | 0 | 90.48 |
Nuclei such as 4He, 12C, 16O, and 20Ne that contain an even number of both protons and neutrons have no net spin. This suggests that both protons and neutrons can pair, in much the same way that electrons pair.
Neutrons do not pair with protons. Deuterium (2H), for example, has an unpaired proton and an unpaired neutron, for a spin quantum number of 1.
Protons or neutrons don't always pair when we might expect them to. 7Li contains three unpaired protons and 10B contains three unpaired protons and three unpaired neutrons.
The spin of a nucleus becomes important in the presence of a magnetic field. Consider a compound that contains hydrogen, for example. One-half of the 1H nuclei in the sample will spin in a direction that produces a tiny magnetic field aligned with the external magnetic field. The other half will spin in a direction that generates a tiny magnetic field opposed to the laboratory magnet. The result is a small difference between the energies of the two spin states of these nuclei. We can measure this difference by irradiating the sample with radiowaves that carry just enough energy for the spins of the 1H nuclei to flip over, from +1/2 to -1/2 and vice versa.
This experiment has three important characteristics. First, the energy is absorbed by
the nuclei. Second, the experiment must be done in a magnetic field. Finally, the
absorption occurs when the system is in resonance ![]() when the energy of the radiation is exactly equal to the difference between the
energies of the two spin states. The experiment is therefore known as nuclear
magnetic resonance (NMR) spectroscopy.
when the energy of the radiation is exactly equal to the difference between the
energies of the two spin states. The experiment is therefore known as nuclear
magnetic resonance (NMR) spectroscopy.
The NMR experiment can be done with any nucleus that has a spin quantum number that is not zero. At first glance, this experiment would seem to be of interest only to physicists, to probe the properties of a nucleus. Shortly after the NMR phenomenon was observed, however, it was found that the chemical environment of the nucleus influences the frequency at which it absorbs radiation.
When the author of this section was a graduate student, he studied the 13C NMR spectrum of the following compound. (G.M. Bodner and L. J. Todd, Inorg. Chem., 13, 360-363 (1974).)

The spectrum he obtained is shown in the figure below. There are seven lines in this
spectrum. Reading from left to right, the first line can be assigned to the CO groups
bound to the chromium atom. The next line corresponds to the carbon atom on the benzene
ring that carries the ![]() OCH3
substituent. The three lines of relative intensity 2:1:2 were assigned to the carbon atoms
meta, para, and ortho to the substituent, respectively. The
last two signals in this spectrum can be assigned to the carbon atoms in the
OCH3
substituent. The three lines of relative intensity 2:1:2 were assigned to the carbon atoms
meta, para, and ortho to the substituent, respectively. The
last two signals in this spectrum can be assigned to the carbon atoms in the![]() OCH3 substituent and the CH2Cl2
solvent.
OCH3 substituent and the CH2Cl2
solvent.

Because of the wealth of information in a 13C NMR spectrum, this soon became one of the most powerful techniques in the chemists' repertoire to identify the product of a chemical reaction or to determine the structure of a substance isolated from natural sources.
In recent years, NMR spectroscopy has lead to a revolution in the practice
of diagnostic medicine. To understand why, let's consider the relative number of 1H
nuclei in a sample that have a spin of +1/2 versus -1/2.
When the sample is placed in a magnetic field, the energies of the two spin states aren't
quite the same. The difference in the population of these states is relatively small, but
it means that there is a slightly higher probability of a spin flip occurring in one
direction than the other. As a result, there is a net absorption of radiation until the
population of the two spin states becomes the same. If we turn off the radiation, we can
measure how long it takes for the system to relax![]() for the population of the two spin states to return to equilibrium.
for the population of the two spin states to return to equilibrium.
In 1971 a remarkable discovery was made: The relaxation time for the 1H nuclei in the water in malignant tissue in rats was significantly longer than normal tissue. As additional experiments were carried out it became apparent that the difference between the relaxation times of water in different environments was large enough to distinguish between gray matter and white matter in the brain, which appear virtually identical in x-rays or CAT scanes.
Before this technique could be used for medical diagnosis, spectrometers had to be developed that could scan objects as large as the human body. Because it involves using NMR to produce images of the tissue within the body, this technique was initially called NMR imaging. It soon became apparent that people were scared by the term nuclear in the phrase nuclear magnetic resonance imaging. The technique therefore became known as MRI. MRI is now used routinely to obtain the kind of information that previously could only be obtained by exploratory surgery.
The diverse role that MRI will play in biomedical research in the 1990s can be appreciated by noting that MRI has recently been used to measure the effect of age on the rate of flow through the aortic system [Journal of Applied Physiology, 74, 492-7 (1993)], to map the human visual cortex [Proceedings of the National Academy of Sciences, 89, 11069-73 (1992)], and to study the effect of sensory stimulation on the brain [Proceedings of the National Academy of Sciences, 89, 5951-5 (1992)].
Organic Chemistry: Structure and Nomenclature of Hydrocarbons
Structure and Nomenclature of Hydrocarbons | Isomers | The Reactions of Alkanes, Alkenes, and Alkynes | Hydrocarbons | Petroleum and Coal | Chirality and Optical Activity
Periodic Table | Periodic Table | Glossary | Cool Applets
Gen Chem Topic Review | General Chemistry Help Homepage | Search: The general chemistry web site.